Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“Aging is the price we pay for the privilege of living.“
Welcome, fellow traveler.
I’m glad you’ve found your way here. Somewhere along the way, maybe in a quiet moment before sleep, or while watching someone you love grow older, you’ve probably asked yourself: Why do we age? Why can’t the body remain in that luminous, effortless state of youth?
From one perspective, aging is simply the passing of years, a slow surrender to entropy. But look closer, and you’ll see it is far more intricate… a silent choreography written deep into the fabric of our biology. Aging is not a single event, but a symphony of changes unfolding cell by cell, molecule by molecule. And like any symphony, it has a score. Science calls the notes in that score the hallmarks of aging: twelve interwoven forces that shape the story of our lives.
These hallmarks are not just the authors of wrinkles or gray hair. They are the hidden architects of memory, vitality, and resilience. They govern the slow fraying of tissues, the dimming of cellular energy, and the subtle shifts that open the door to chronic disease.
Here, we will explore them not as dry scientific concepts, but as a living map, a way to navigate the terrain between birth and death, between vigor and decline. Each hallmark is a doorway into a deeper understanding of ourselves. And while they are all tangled together, they also hold clues for how we might slow the tempo, or even change the melody, of our own aging.
Because longevity is not really about defying time, it’s about shaping our time. It’s about adding more life to the years we have.
So whether you come here seeking answers, practical tools, or simply to satisfy a restless curiosity, welcome to this journey. Let’s begin.
Below you’ll find the table of contents, a map for the road ahead.
That’s our starting point. From here, we’ll break down what each of these means, why they matter, and what the science says we can do about them. Ready? Let’s dive in !
Let’s start at the source… the Primary hallmarks of aging. These are the first cracks in the foundation, the root causes that kick off the entire process of biological aging.
At this early stage, the damage begins quietly. It’s subtle but powerful, like a ripple that becomes a wave. This is where cellular aging really takes hold, long before we notice gray hairs or stiff joints.
These primary hallmarks include:
Together, these issues set the stage for everything that comes next.
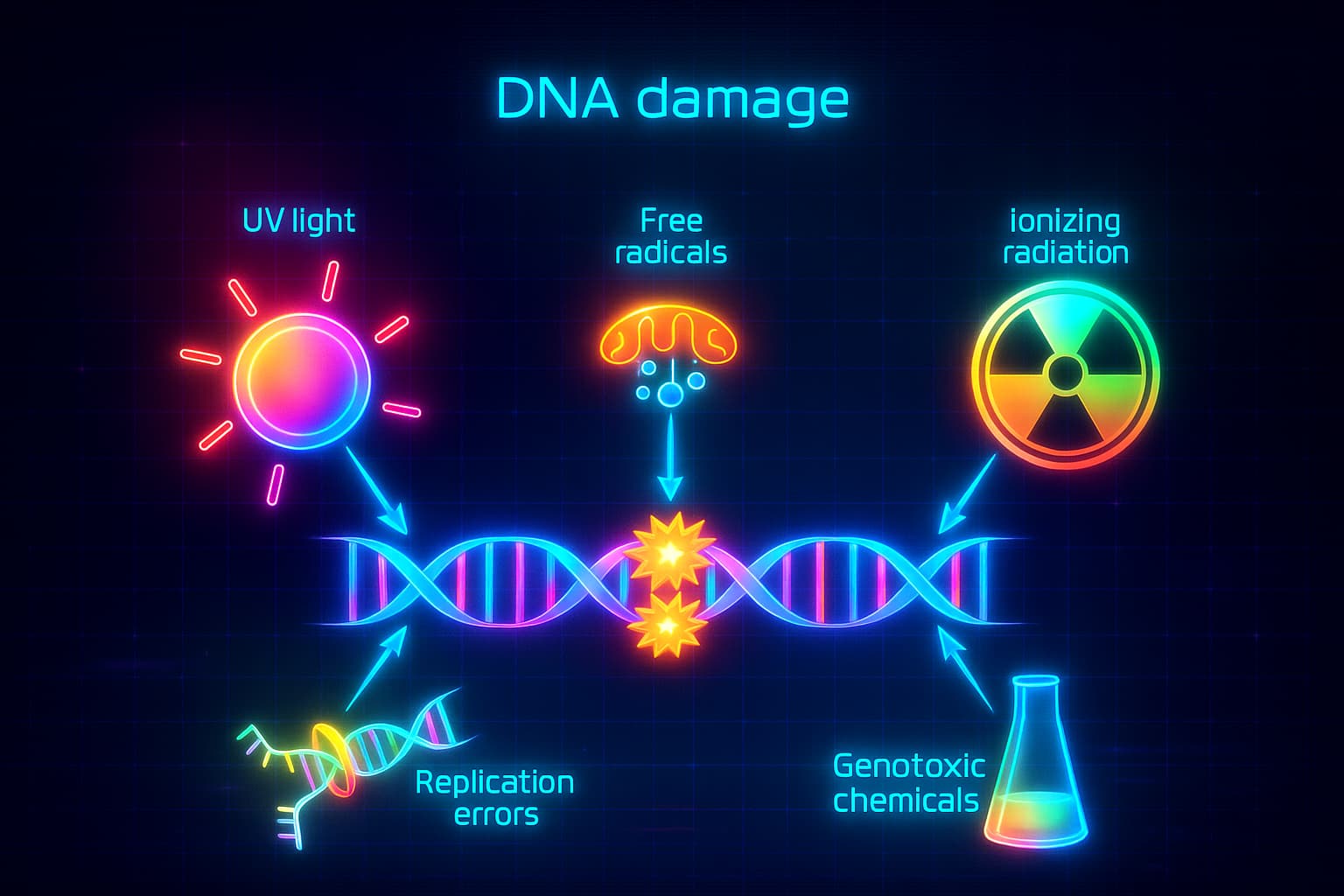
Imagine your body is a house, not a static structure, but a living, breathing one that’s always under renovation. At the heart of it is your blueprint: DNA.
This DNA blueprint guides your cells, telling them when to grow, repair, and renew. But over time, just like a well-used manual, it starts to wear out. Exposure to sunlight, toxins, stress, and even normal metabolism causes small breaks and smudges in the code.
At first, it’s not a big deal, our cells have brilliant built-in repair crews. They patch up errors, restore order, and keep your biology humming.
But as we age those repair teams get slower and less precise. Fixes take longer. Mistakes get missed. And little by little, damage builds up. Doors creak. Pipes leak. The house still stands, but it’s not what it used to be.
This is genomic instability: a central hallmark of aging and one of the key answers to the question: why do we age?
And why this matters for longevity? if we can understand how and why DNA damage accumulates, we can start to explore ways to reduce it, repair it faster, or even prevent it altogether. That’s the kind of science that moves us from aging as a mystery… to aging as something we can influence.
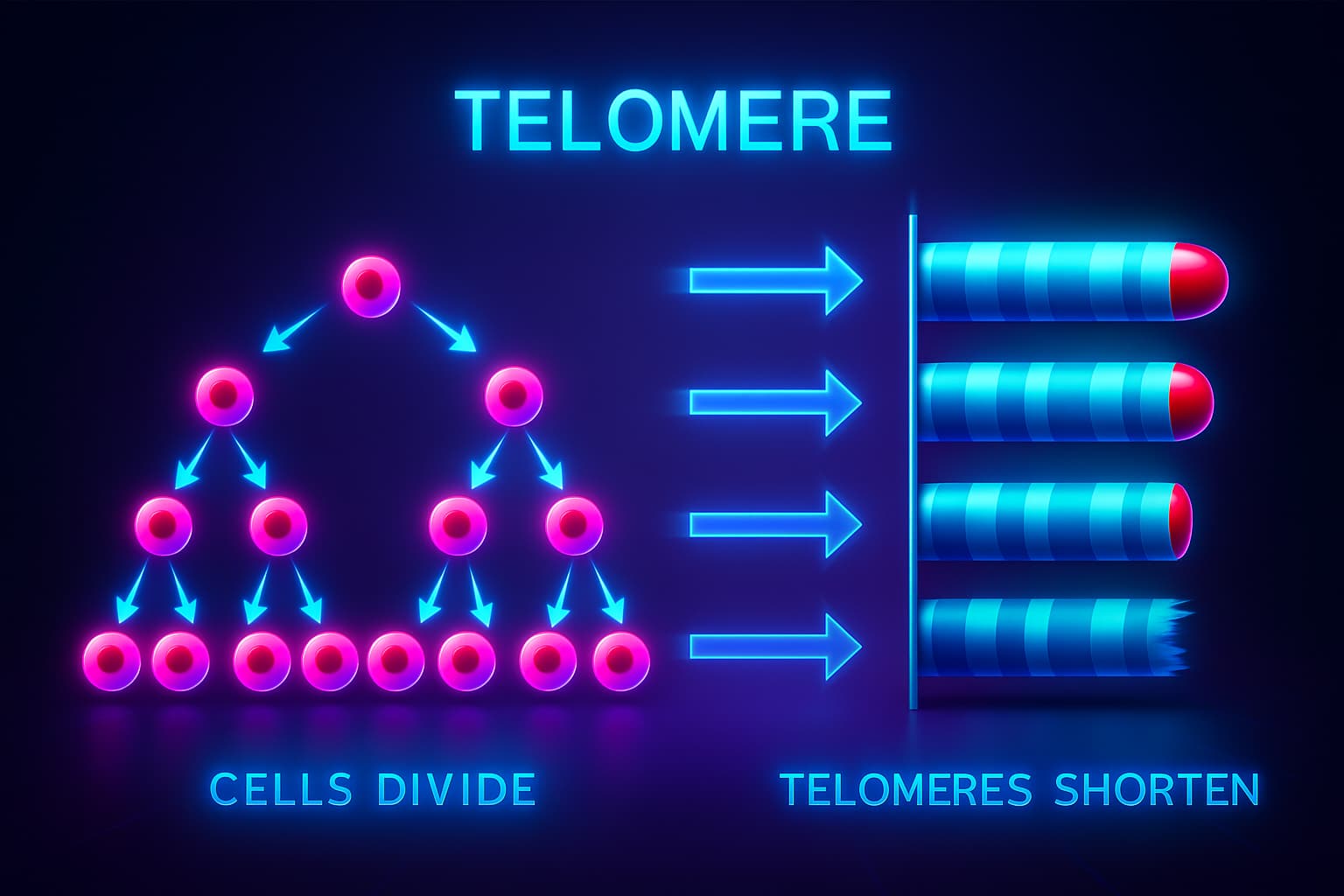
Let’s return to our DNA, the instruction manual we talked about earlier. At the very ends of each DNA strand are protective caps called telomeres. If DNA is your cellular shoelace, then telomeres are the little plastic tips that keep it from fraying.
But these caps do more than just tidy things up: they act like a biological helmet, shielding your chromosomes from damage during cell division.
Every time your cells divide (which they do constantly to keep your tissues fresh and functional), a tiny bit of the telomere gets shaved off. It’s like photocopying a book, the edges blur just a little more each time.
Some cells, like embryonic stem cells, come equipped with a special enzyme called telomerase, think of it as a master restorer that rebuilds these tips and keeps them intact. But in most of our adult cells? That enzyme is barely active. So over time, the telomeres shorten, again and again.
Eventually, they wear down to the point where they can no longer protect the DNA. At that moment, the cell senses danger, and may enter a state of dysfunction, stop dividing altogether, or even self-destruct. This contributes directly to cellular aging, and it’s one of the reasons tissues lose their ability to heal and renew as we get older.
This process is called telomere attrition, and it’s a defining feature of the hallmarks of aging. It helps answer the big question of why we age, offering insight into how biological time ticks away at the very core of our cells.
The good news? Scientists are exploring ways to support telomere health through lifestyle, supplements, and even experimental gene therapies. It’s still early days, but understanding telomere shortening is a key step toward unlocking new tools for healthy longevity.
Remember that blueprint we talked about? It turns out, it’s even more brilliant, and more complicated, than we thought.
Your DNA doesn’t just contain instructions for your whole body, it holds the full architectural plan for every part of your biological house: every room, every wire, every pipe. But not every room needs the entire blueprint.
A kitchen doesn’t need the plans for the bedroom. A heart cell doesn’t need to know how to make stomach acid.
So how do your cells know which parts of the blueprint to read?
That’s where epigenetics comes in: the system of molecular “sticky notes” and switches that helps each cell know what to pay attention to, and what to ignore. Thanks to epigenetics, a skin cell stays a skin cell, and a brain cell never suddenly decides to act like a liver cell.
These sticky notes are actually small chemical tags called methyl groups. They attach to specific parts of your DNA and tell the cell, “Don’t read this section,” or “Turn this page on.” It’s how your body maintains cellular identity and function without rewriting the underlying code.
But here’s where biological aging kicks in.
As we age, this once-precise system starts to break down. Methylation patterns become messy. The wrong pages get shown. Some important sections get silenced. And over time, cells become confused: they lose their identity, stop functioning properly, or even behave in harmful ways.
This breakdown is known as epigenetic alteration, and it’s one of the most revealing hallmarks of aging. It explains why we age not just from damage, but from a gradual loss of control over how our cells express themselves.
What’s truly fascinating is that these epigenetic changes can be measured. Scientists can now estimate your biological age: the age your body acts like based on your DNA methylation patterns. And in some cases, interventions like diet, sleep, and even reprogramming factors have shown promise in slowing or even reversing this epigenetic drift.
In other words, if we can better understand and manage these sticky notes, we may just have a powerful tool for boosting longevity from the inside out.
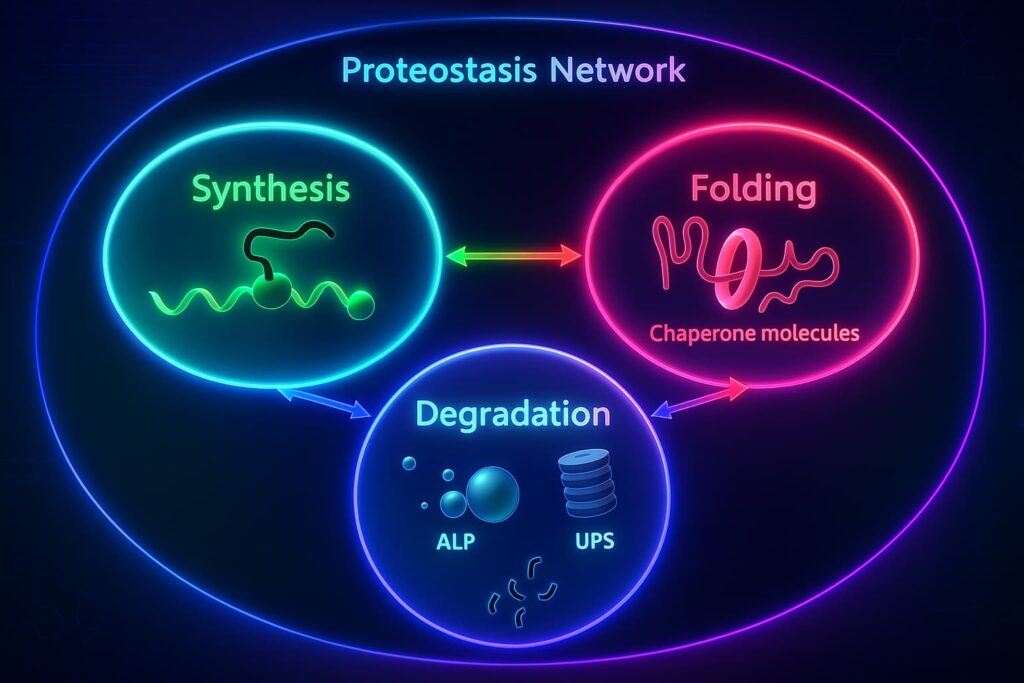
Let’s talk about the unsung heroes inside your cells: proteins.
Proteins are like the full-time staff in your biological house, folding into precise shapes to manage thousands of essential tasks. They build structures, carry messages, repair damage, and even clean up after themselves.
Yes, you read that right. some proteins are part of the cellular cleanup crew. These include chaperones (which help other proteins fold correctly) and proteasomes (which act like molecular shredders, breaking down damaged or misfolded proteins before they cause trouble).
But as we get older, the system starts to slip.
Everyday wear and tear, oxidative stress, and environmental toxins generate more protein damage than the cleaning team can handle. Misfolded proteins pile up. The cellular housekeeping system known as proteostasis becomes overwhelmed.
Imagine clutter piling up in every room. Dishes in the sink, dust on the floor, broken appliances left unattended. The house still stands, but it’s not running the way it used to.
This breakdown is called the loss of proteostasis, and it’s a major player in biological aging. When protein balance falters, cells lose their efficiency, signaling gets scrambled, and dysfunction starts to spread.
This hallmark doesn’t just explain why we age, it connects directly to age-related diseases like Alzheimer’s and Parkinson’s, where misfolded proteins form toxic clumps in the brain. It’s a clear example of how cellular aging impacts our whole-body health.
Understanding and supporting proteostasis through lifestyle, fasting, supplements, or even cutting-edge therapies may hold the key to healthier, longer lives. After all, longevity starts with a clean, well-functioning house.
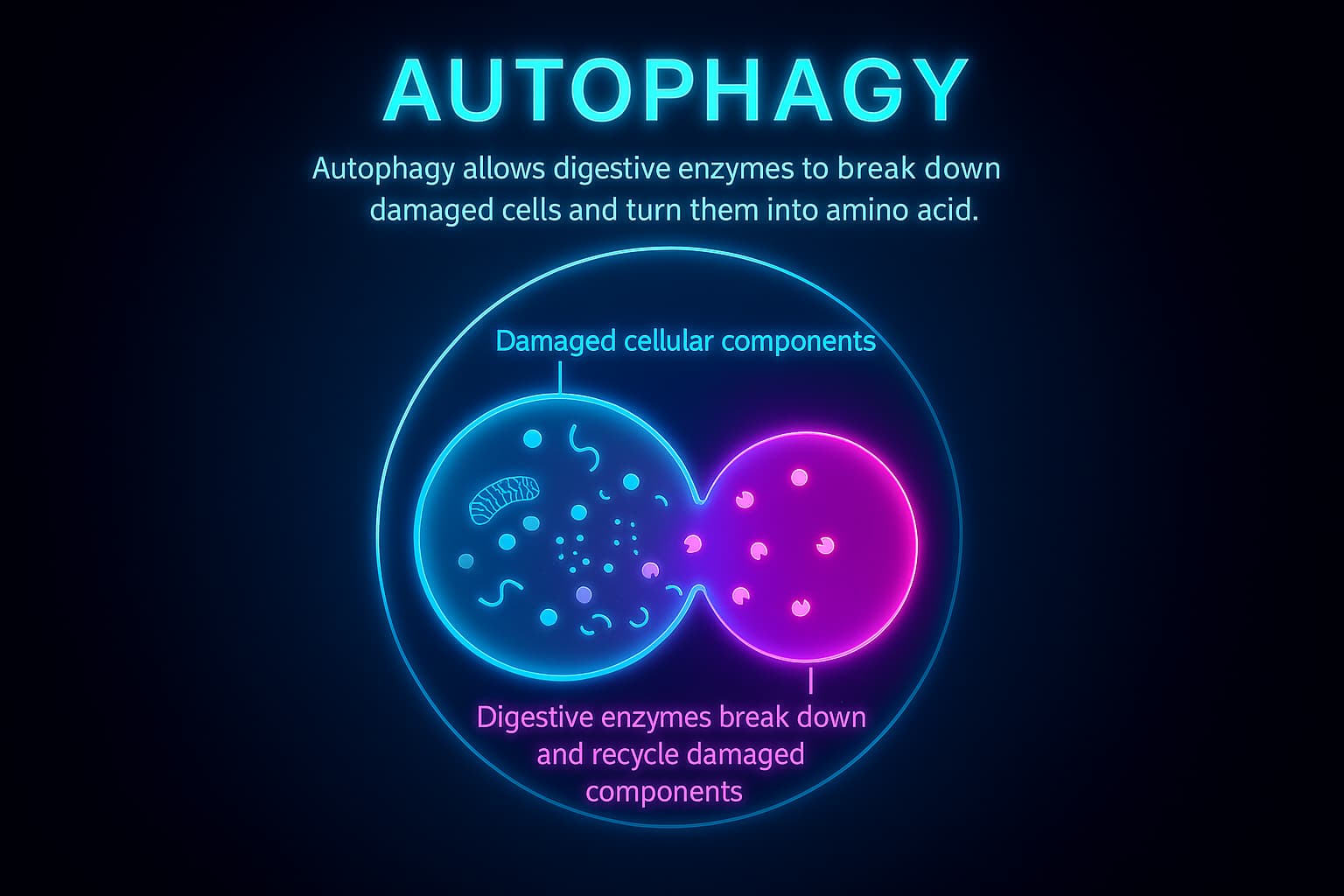
Beyond the cleaning crew of proteins, your cellular house has a full-on recycling system and it’s called autophagy.
While the chaperones and proteasomes handle small messes (like broken or misfolded proteins), autophagy takes care of the heavy lifting. Think of it as the deep-cleaning, junk-hauling service. Its job? To break down and recycle larger waste like damaged mitochondria (your cell’s power generators), busted organelles, or massive debris piles that no other system can handle.
It’s essential, elegant, and incredibly efficient when it’s working well.
But with age, this recycling system begins to stall. The pace slows. The junk piles up. And suddenly, the cell is crowded with clutter, like a garage overflowing with broken appliances and rusty tools. This makes it harder for the cell to function, adapt, and respond to stress.
This decline is called disabled macroautophagy, and it’s a major hallmark of aging. As autophagy weakens, cellular aging speeds up leading to reduced energy, increased inflammation, and a loss of cellular resilience.
Autophagy isn’t just about cleanliness it’s about survival. Cells that can recycle their waste are more adaptable, more efficient, and more likely to stay healthy over time. That’s why autophagy and aging are so closely linked and why boosting autophagy is a hot topic in the longevity world.
Fasting, certain types of exercise, and even compounds like spermidine and rapamycin are all being explored as ways to jumpstart this ancient recycling system.
In short: when the cleanup slows down, aging speeds up. But by understanding and supporting autophagy, we may be able to clear the path toward a longer, healthier life.
Antagonistic Hallmarks: When Defense Turns Dangerous
Now we enter the next phase: the Antagonistic hallmarks of aging.
Unlike the Primary hallmarks which kick off the aging process these are the body’s responses to that damage. At first, they’re protective. Helpful. The body trying to restore balance.
But with time, these adaptive responses go off course. Instead of helping, they start driving aging forward turning into problems themselves.
Let’s imagine your cells as tiny engines each with its own fuel gauge.
These gauges are part of a nutrient-sensing system that tells the body when to grow, when to store energy, and when to shift into repair mode. The system relies on master regulators like insulin, IGF-1, mTOR, and AMPK all working together to keep your body in metabolic balance.
In youth, this system is sharp. It knows when to burn fat, when to build muscle, and when to pause and reset.
But with time and the creeping effects of biological aging that precision starts to blur.
The fuel gauges become faulty. They misread signals, telling the body to store energy when it should be burning it, or ignore real overload, leading to insulin resistance, fat accumulation, and rising blood sugar levels. It’s like pressing the gas and brake pedals at the same time confused cells, chaotic outcomes.
This is called deregulated nutrient sensing, and it’s a classic Antagonistic hallmark of aging. It begins as a protective response to cellular stress but over time, it becomes part of the problem, accelerating cellular aging and disrupting energy balance throughout the body.
The silver lining? We can influence these pathways. Through fasting, exercise, and certain nutrients or therapies, it’s possible to recalibrate these aging sensors keeping the body responsive, efficient, and on the path to healthy longevity.
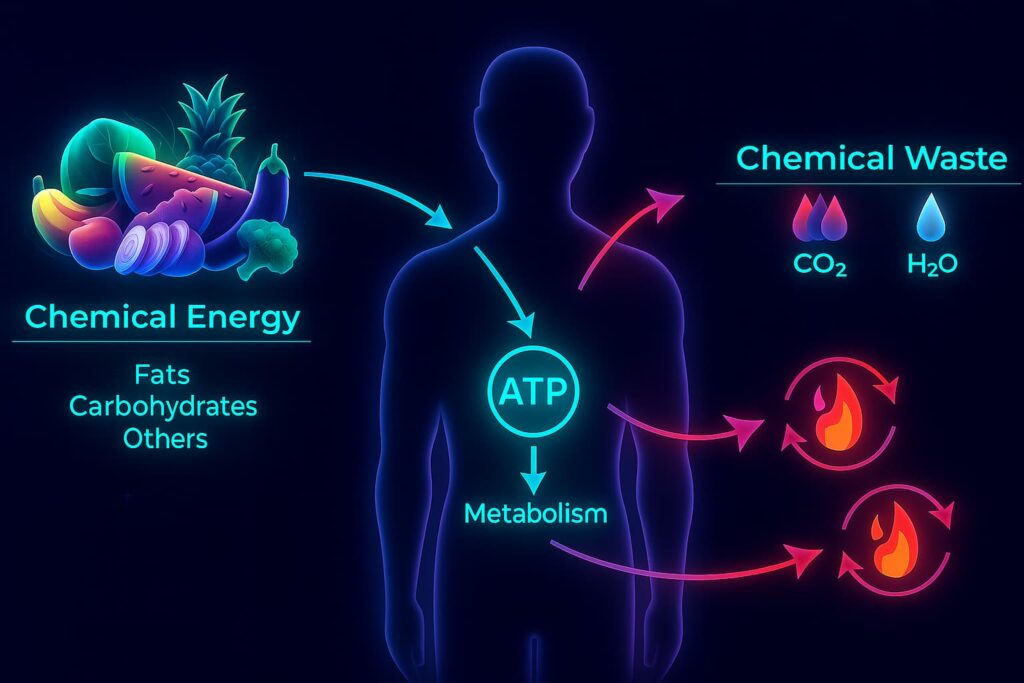
Now let’s talk energy, because every cell in your body runs on it.
Inside each of your cells are mitochondria, tiny organelles often called the powerplants of the cell. Their job? Convert the food you eat into ATP (the energy currency that powers everything from thinking and breathing to muscle contractions and immune defense).
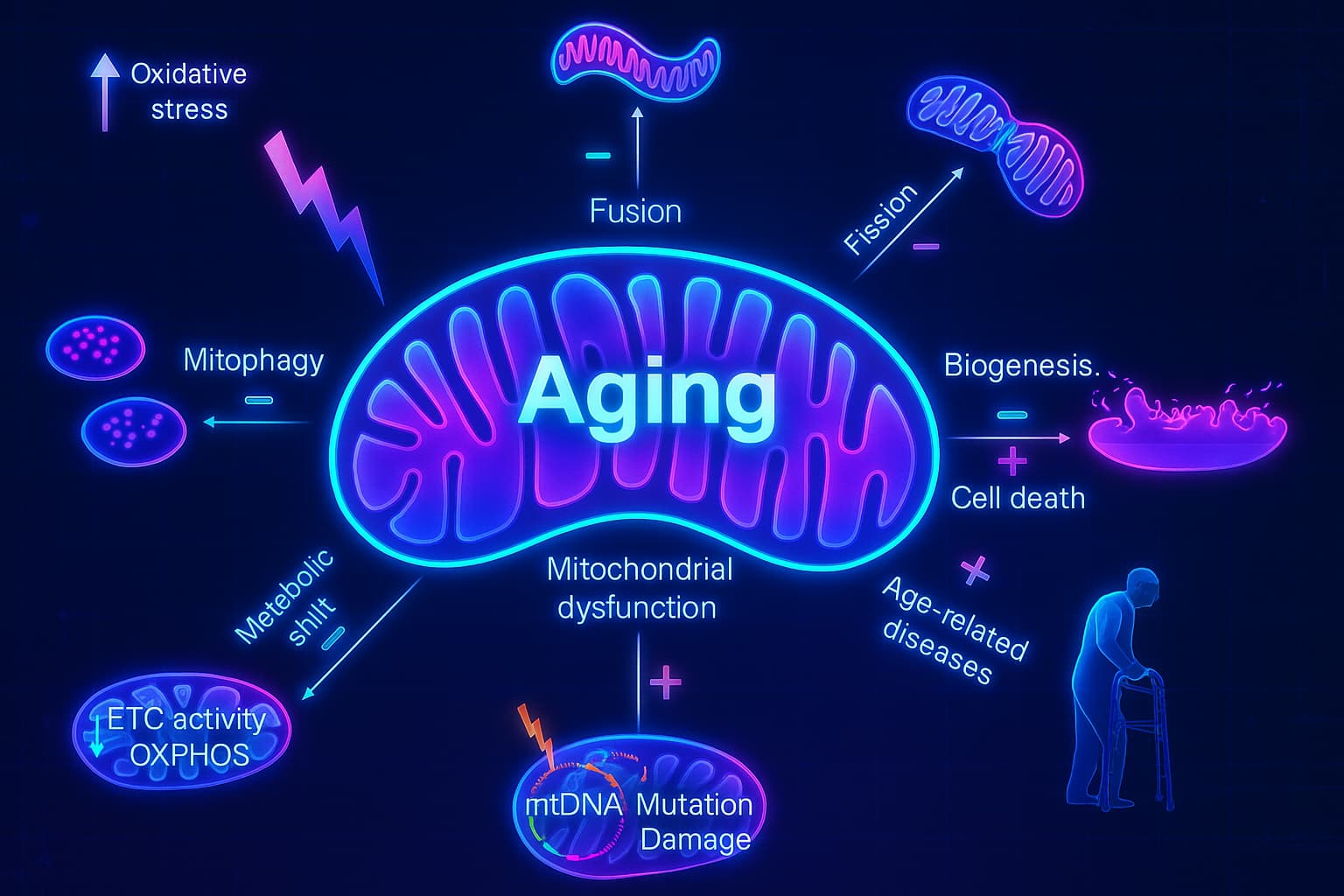
But with age, these energy factories start to break down.
The mitochondria become less efficient. They churn out less ATP and begin to leak harmful byproducts called reactive oxygen species (ROS): unstable molecules that act like smoke from an aging engine. In small amounts, ROS are manageable. But when the smoke builds up, it damages the very machinery meant to keep things running.
This cascade less energy, more damage, contributes to fatigue, muscle weakness, and organ decline. It’s a core driver of cellular aging, and it shows up across everything from neurodegeneration to cardiovascular disease.
So what starts this mitochondrial meltdown?
It’s often triggered by earlier hallmarks of aging, like genomic instability and DNA mutations. When the genes responsible for mitochondrial maintenance take a hit, the powerplants begin to fail and the damage starts to spread.
This is mitochondrial dysfunction, a textbook example of an Antagonistic hallmark of aging. It begins as a necessary function like energy production, but over time, the stress and byproducts of that very process become part of the problem.
The upside? Mitochondria are surprisingly adaptable. Through exercise, calorie restriction, and emerging mitochondrial-targeted therapies, we may be able to boost their performance, lighting the way toward greater longevity and vitality.
The final Antagonistic hallmark of aging brings us to one of the most fascinating and frustrating processes in the aging story: cellular senescence.
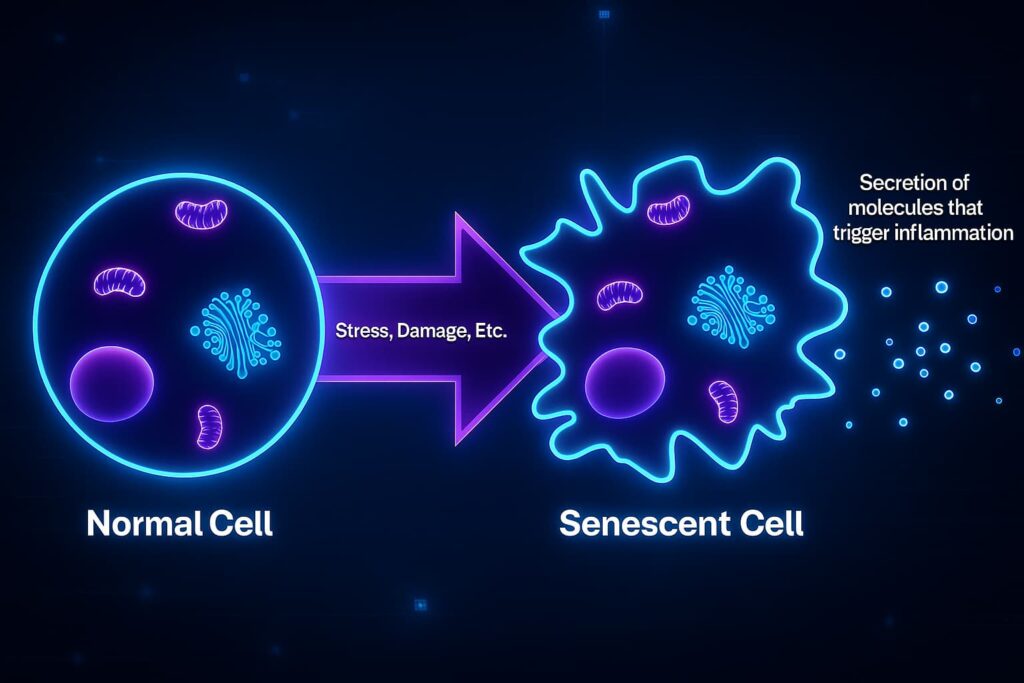
When a cell becomes too old or damaged, it doesn’t always die or get recycled. Instead, it enters a state scientists call senescence, and I like to call it “zombie mode.”
Imagine a worker who’s supposed to retire. Instead of leaving, they just sit at their desk, doing nothing… but still taking up space, blocking others from working, and occasionally shouting confusing instructions. That’s what a senescent cell does, it stops dividing, stops functioning, and starts sending out inflammatory signals that mess with its healthy neighbors.
Originally, cellular senescence was meant to be helpful. It’s the body’s way of preventing damaged cells from dividing and becoming cancerous. A built-in safety mechanism.
But over time, as more and more cells enter zombie mode, the tissue environment turns toxic. Chronic inflammation sets in. Repair slows down. And this contributes directly to cellular aging, tissue decline, and age-related disease.
What triggers this transformation? Often it’s earlier Primary hallmarks like DNA damage, telomere shortening, or genomic instability that tell the cell, “It’s too risky to keep dividing.”
So once again, we see the pattern: a system designed to protect us in youth becomes harmful in later life. That’s what makes cellular senescence a textbook Antagonistic hallmark of aging part of why we age, and a key target for emerging longevity therapies like senolytics, which aim to clear out these zombie cells for good.
We’ve explored the root causes (the Primary hallmarks) and the body’s adaptive but sometimes harmful responses (the Antagonistic hallmarks).
Now we arrive at the final group: the Integrative hallmarks of aging, or as I like to call them, the manifestations of aging.
These are the big-picture consequences: what happens when cellular damage piles up and our repair systems falter. This is where biological aging starts to show in ways we can feel: slower healing, lower resilience, and a general sense that things just aren’t working quite like they used to.
In short: this is where all the upstream damage becomes real-world decline.
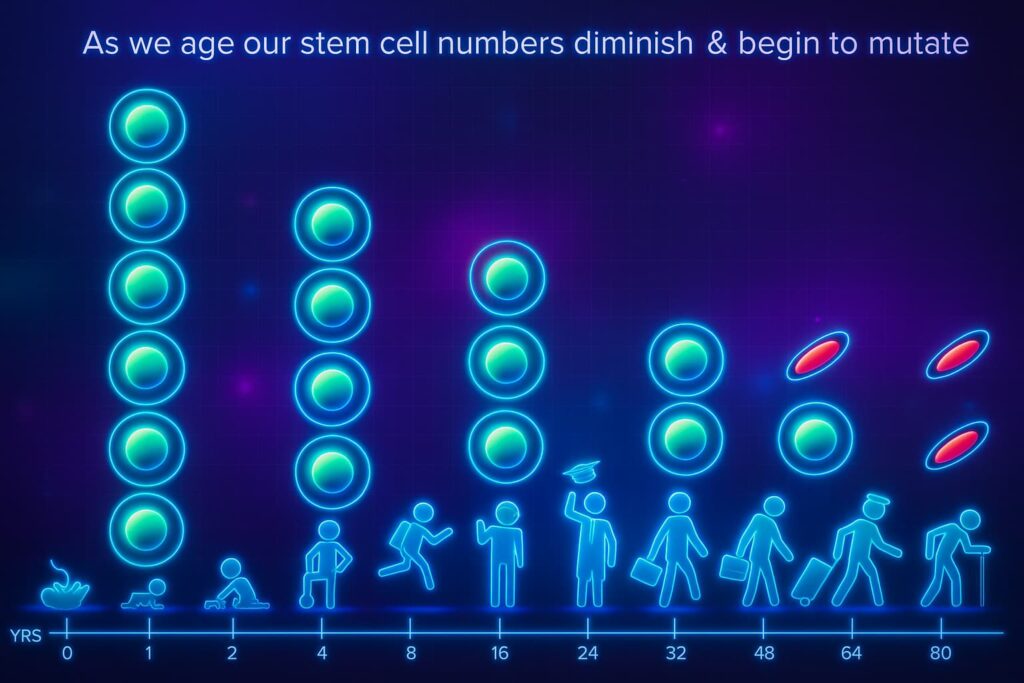
Let’s talk about your body’s built-in repair crew: stem cells.
Stem cells are special because they haven’t chosen a career yet. they’re blank slates, waiting on the sidelines to become skin cells, blood cells, muscle cells, or whatever your body needs at the moment. They’re essential for tissue regeneration, healing, and long-term vitality.
When you’re young, the bench is full. Stem cells are plentiful, active, and ready to jump in whenever damage occurs.
But as cellular aging progresses, this backup team starts thinning out.
Earlier hallmarks of aging like DNA damage, telomere shortening, and chronic inflammation gradually wear out the stem cell population. Some stem cells stop dividing. Others enter senescence (that zombie mode again). And new recruits? Fewer and farther between.
This decline is known as stem cell exhaustion, and it’s one of the most visible signs of biological aging. It’s why injuries heal slower, skin thins, and the immune system weakens with age.
When the repair team runs out, maintenance slows to a crawl and the whole system becomes more fragile.
Understanding how to preserve and revive stem cell function is one of the most exciting frontiers in longevity science. Because when we protect the repair crew, we protect the entire structure.
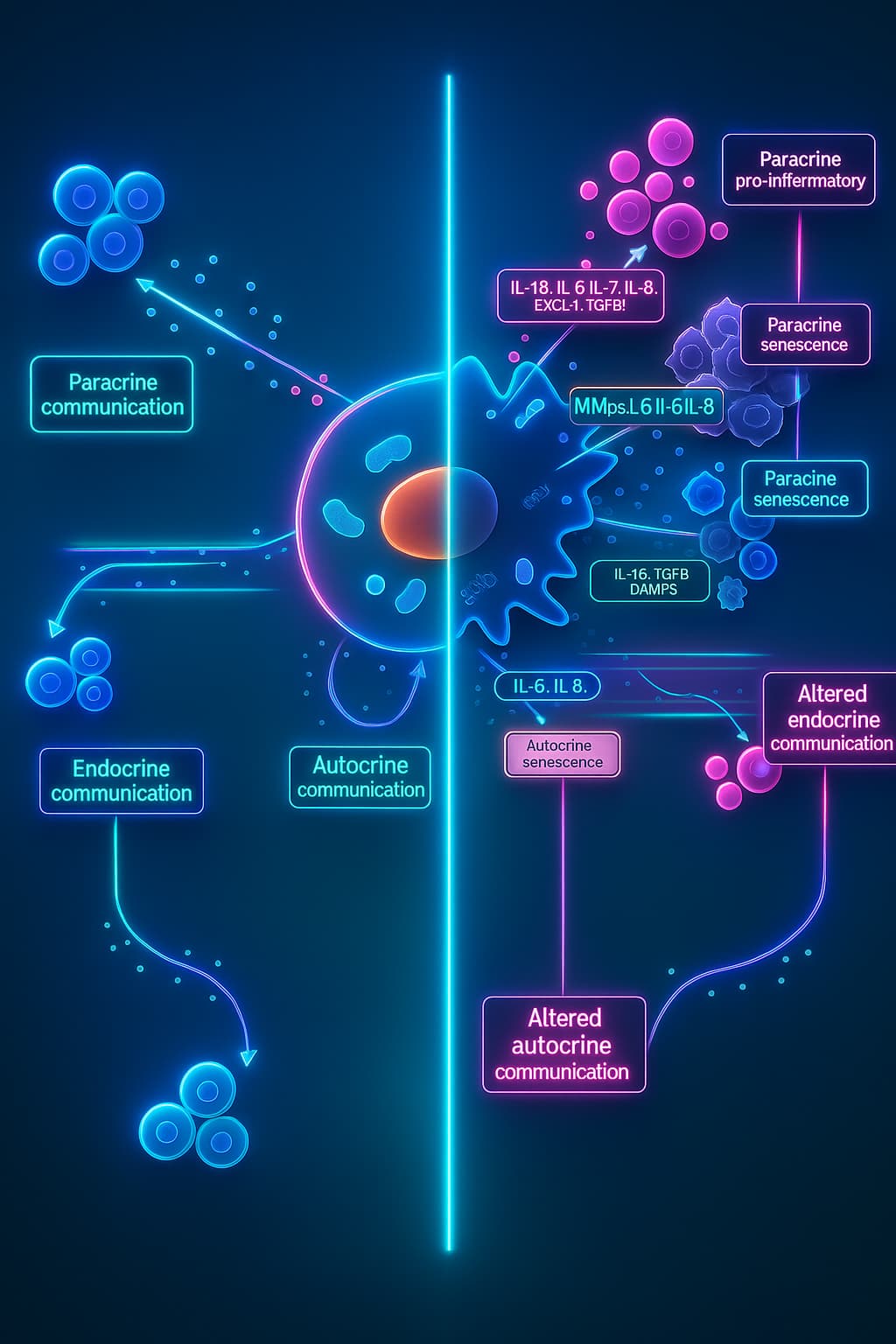
In healthy tissues, cells are constantly chatting sending signals, coordinating actions, and working together like a well-synced orchestra. Growth, repair, immune defense. it all relies on clear communication between cells.
Think of it like a giant group chat where everyone knows their role and responds in real time.
But with age, the messages start to glitch.
Some cells stop listening. Others send out garbled signals, or worse: inflammatory messages that confuse or damage their neighbors. The group chat turns into a mess of noise, and the coordination that once kept everything running smoothly begins to fall apart.
This breakdown is called altered intercellular communication, and it’s one of the most far-reaching hallmarks of aging. It doesn’t just affect one cell type, it affects the entire tissue ecosystem.
What causes it? The same culprits we’ve met before: accumulated cellular damage, oxidative stress, and especially the influence of senescent cells. These “zombie cells” release harmful factors that interfere with healthy cell signaling, creating an inflammatory environment that disrupts healing, accelerates disease, and promotes cellular aging.
In a way, this is where aging truly becomes more than just damage it becomes dysfunction.
Because aging isn’t just about individual cells wearing out, it’s about the whole network losing connection. When the system can’t coordinate, the body loses resilience. That’s what makes altered cell-to-cell communication a powerful driver of biological aging and a key target for future longevity therapies.
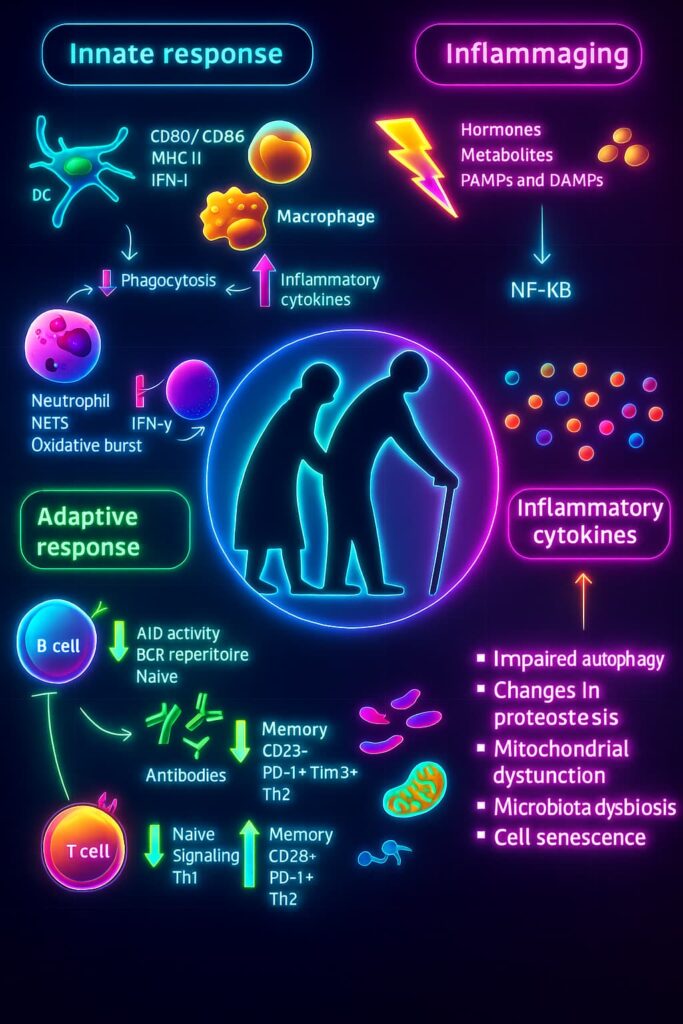
Let’s talk about inflammation. your body’s built-in alarm system.
When you get injured or sick, your cells sound the alarm by releasing chemical messengers called cytokines. These attract immune cells, kickstart repairs, and help the body heal. Once the danger passes, the alarm turns off and everything goes back to normal.
That’s how it’s supposed to work.
But with aging, this system starts to misfire.
All the earlier issues :DNA damage, misfolded proteins, zombie-like senescent cells. keep tripping the alarm, even when there’s no threat. It’s like a smoke detector that won’t stop beeping long after the toast is fine.
This leads to chronic inflammation, a state where the immune system is constantly activated, flooding the body with low-level chemical signals. Instead of helping, this steady trickle of immune activity starts damaging healthy tissues, contributing to problems like tissue breakdown, fibrosis, and the progression of chronic diseases.
This constant background noise is often called inflammaging and it’s one of the most insidious hallmarks of aging.
It doesn’t just exhaust your immune system it accelerates cellular aging, disrupts tissue function, and raises your risk for everything from heart disease and arthritis to Alzheimer’s and type 2 diabetes.
The good news? Lifestyle choices like anti-inflammatory diets, exercise, quality sleep, and even certain supplements (like omega-3s, curcumin, or resveratrol) can help turn down the volume and may become key tools for boosting longevity.
Because when the fire that won’t go out finally cools, the body gets a chance to heal, restore, and thrive again.
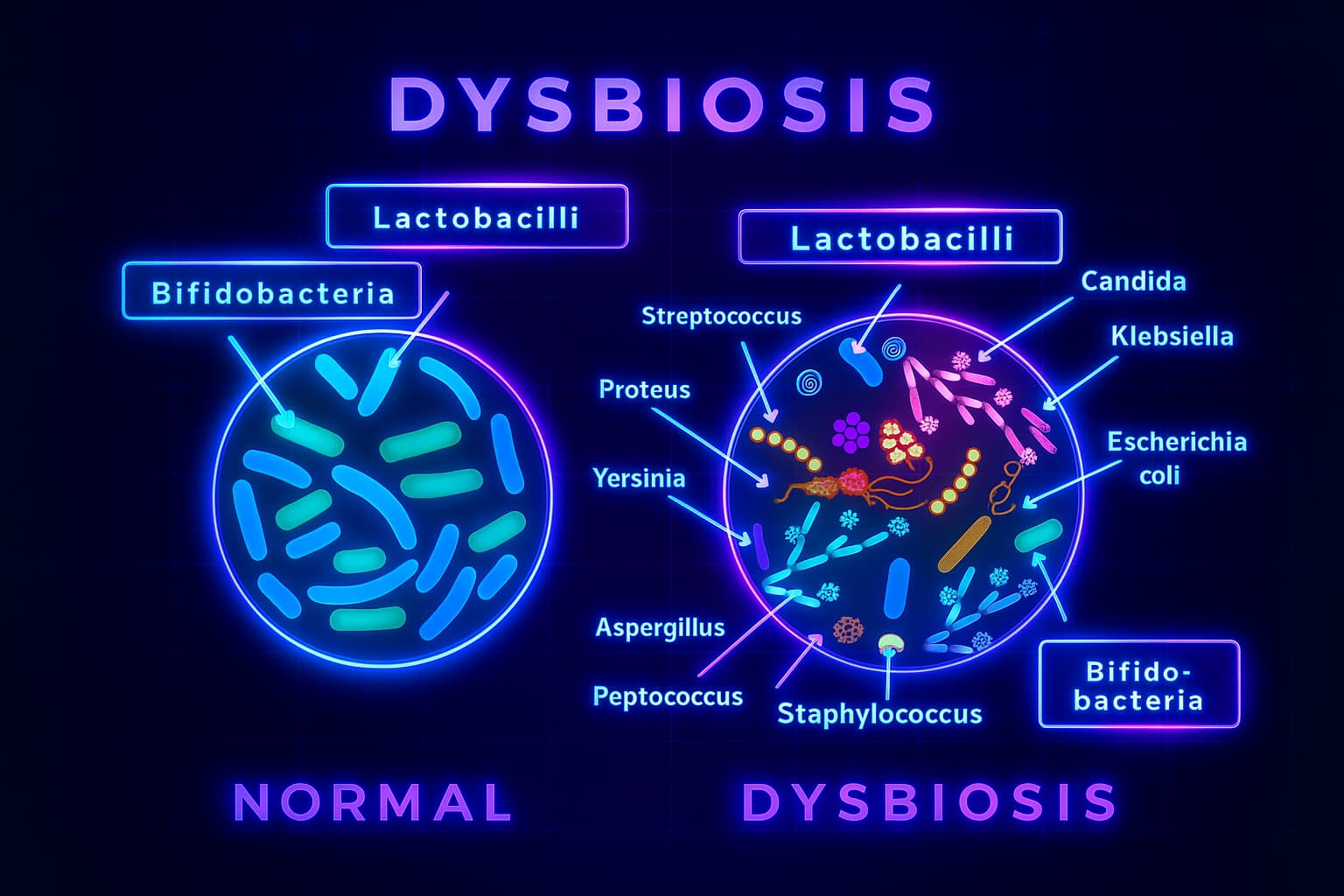
We’ve made it to the final hallmark of aging and it’s one that starts in your belly: dysbiosis, or the imbalance of your gut microbiome.
Picture your gut as a vibrant garden: lush, diverse, and teeming with helpful microbes that support digestion, regulate immunity, and even influence your mood and brain health. These microbial allies are essential for keeping the whole system running smoothly.
But as we age, this garden starts to change.
The healthy diversity fades. The soil becomes depleted. And harmful bacteria, the weeds, begin to spread. This microbial imbalance, or dysbiosis, crowds out the beneficial species we rely on. The result? Weaker immunity, digestive troubles, and even links to cognitive decline, anxiety, and depression.
And just like many other hallmarks of aging, this shift doesn’t come out of nowhere.
It’s driven by the damage we’ve already explored: chronic inflammation, poor nutrient sensing, weakened repair systems, and lifestyle changes that often accompany aging, like a less varied diet, medications, or reduced physical activity.
In the end, dysbiosis and aging are deeply intertwined. When our microbial garden loses balance, it sends ripple effects throughout the body, another sign that cellular aging is taking root.
But the microbiome is flexible. Through diet, prebiotics, probiotics, fermented foods, and lifestyle tweaks, we can often restore a healthier ecosystem. Because a thriving gut supports not just digestion, but longevity from the inside out.
The Journey Begins Here
If you’ve made it this far, or even just skimmed your way down (hey, no judgment!). you’re probably asking:
What can we actually do with all this information about the hallmarks of aging?
Many of these hallmarks can now be measured, tracked, and even influenced through lifestyle, supplements, and emerging longevity therapies that support healthy aging at the cellular level.
Let’s bring it down to earth:
Lifestyle: Something as simple as intermittent fasting can help reset your body’s nutrient-sensing pathways, the same ones we just explored under deregulated nutrient sensing.
Supplements: Natural compounds like spermidine have been shown to support autophagy, the cellular recycling system that slows down with age.
And this is just the beginning.
This blog is your map , your launchpad into the world of longevity science. Each of the hallmarks we touched on here has its own dedicated deep-dive post where we explore the biology, the risks, and most importantly: what you can do about it.
Because at the heart of it all, our lives and the lives of those we love are the most meaningful things we have.
They deserve to be protected, preserved, and extended with everything we’ve got.
And the most powerful tool we have to do that?
Knowledge.
Knowledge is how we fight aging.
Knowledge is how we defend our health.
Knowledge is how we build a future where vitality doesn’t fade with time, but evolves with it.
So I invite you, deeply and sincerely, to explore the hallmarks that speak to you most. Dive in. Get curious. Ask questions. And let’s take this journey together.
Because to understand aging is to begin mastering it.
And the quest for longevity?
That’s not just a personal mission.
It’s one of the most important things we can do as scientists, as individuals, and as human beings.