Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“The Body Doesn’t Cool Down With Time, It Smolders From Within“
Welcome, keeper of the body’s internal fire.
If you have been traveling with us through the hallmarks of aging, you know aging is not only about what breaks down, but also about what refuses to switch off. Among the most dangerous of these unending processes is chronic inflammation, a slow-burning fire that quietly reshapes health over time.
Not all inflammation is harmful. In its acute form, it is the body’s first responder, rushing to heal wounds, fight infections, and repel invaders. Yet with age, this quick flare of defense can become stuck in the “on” position. The result is a persistent, low-grade immune activation known as inflammaging: a fusion of inflammation and aging, now recognized as one of the most well-studied biological patterns driving decline.
The connection between chronic inflammation and aging is profound. Left unchecked, this constant immune pressure damages tissues, disrupts metabolism, accelerates stem cell exhaustion, and distorts cell-to-cell communication. It is a key contributor to cardiovascular disease, neurodegeneration, and many of the chronic conditions that limit both lifespan and vitality. In the delicate balance of inflammation and longevity, too much fire shortens life, while controlling it may extend the healthy years we have.
In this exploration, we will uncover:
To understand chronic inflammation and aging is to see how a defense meant to protect can become a force that erodes health. And by learning to calm these flames, we may not only slow aging, but also preserve the strength, clarity, and resilience that make life rich.
Let us step into the quiet heart of this fire and learn how to bring it back under control.
Inflammation 101: Your Body’s Built-In Fire Department
Let’s start at the beginning. with the hero.
Inflammation is your body’s emergency response team. Think flashing sirens, rushing medics, fire trucks squealing around corners. The moment something goes wrong: a splinter, a virus, a paper cut inflammation races in.
Your immune system calls the shots: “Trouble ahead!”
White blood cells show up like paramedics. Cytokines (chemical messengers) spread the word like fire dispatchers, telling nearby cells to get involved. Blood vessels widen. Fluid floods the area. Heat and redness bloom. It’s dramatic, but it’s all by design.
In this fast-paced, high-alert state, your body:
This is acute inflammation: powerful, localized, and short-lived. It’s what makes a sprained ankle swell or a flu give you a fever. It’s also what saves your life, over and over again.
In its prime, inflammation is like a disciplined firefighter crew: it shows up fast, does the job, and gets out.
But as we age, that crew starts losing coordination. Some firefighters show up too late. Others refuse to leave. A few even start pouring gas on the fire instead of water.
This is where things start to smolder…
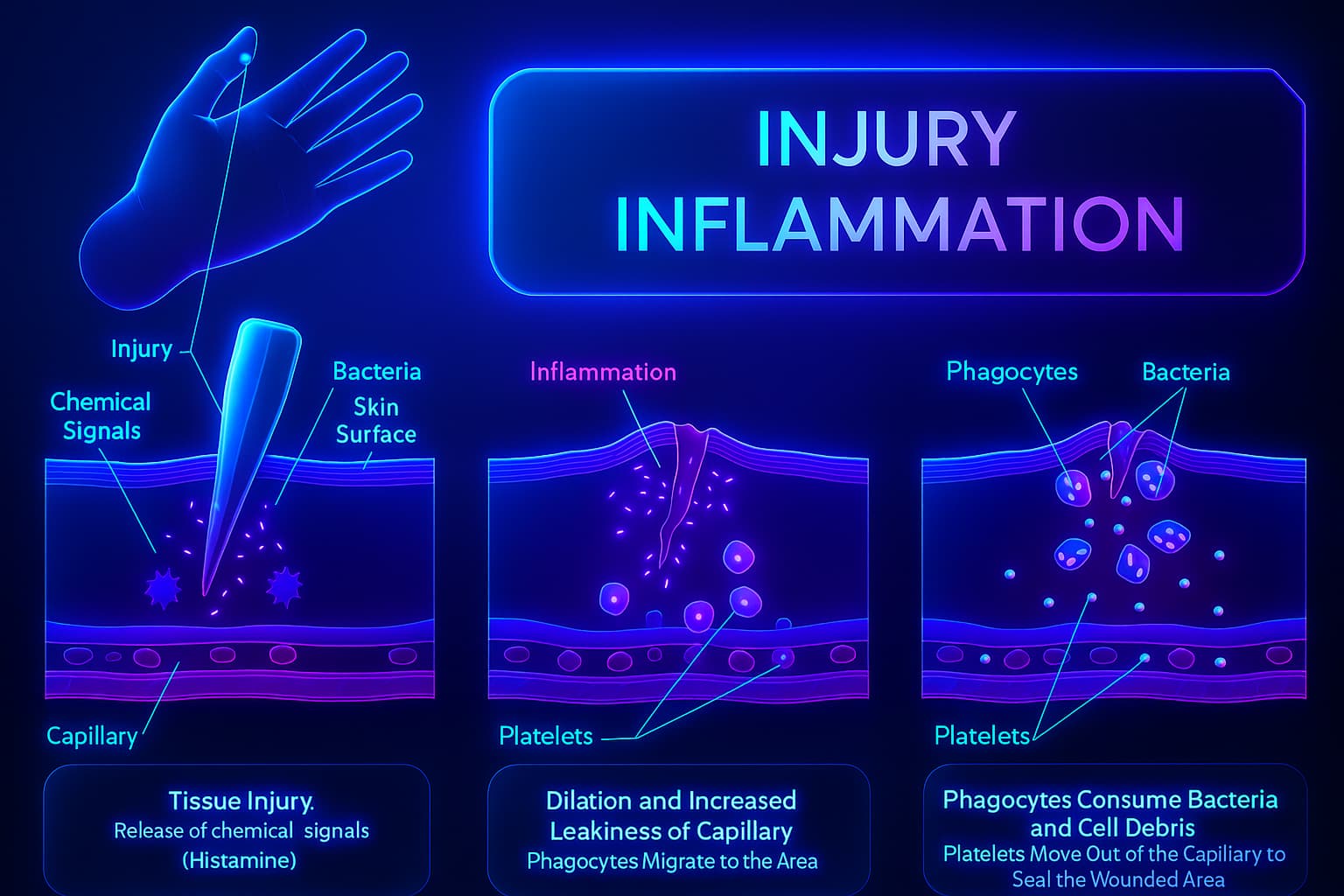
When the Fire Won’t Go Out: Chronic Inflammation and Aging
Now meet the version of inflammation that forgot how to shut up, the one that lingers long after the danger is gone. This is chronic inflammation, and biologically speaking, it’s less like a firefighter and more like a smoke machine someone left running in the basement.
Instead of quick, coordinated bursts of immune activity, chronic inflammation is a slow, background hum of immune noise. It’s not explosive, it’s erosive. Not sharp and painful, just… stuck. It’s the body trapped in alert mode, even when there’s no real threat.
At the cellular level, it’s like your immune system is pacing the hallways at 3 a.m., muttering to itself. Cells that should be quiet are constantly whispering cytokines, tiny chemical messages that say, “Be careful!” or worse, “Attack!” Nearby cells overhear this and ramp up their own defenses. Over time, tissues become saturated in this paranoid chatter, unable to relax or regenerate.
And unlike the well-timed drama of acute inflammation, this kind of immune activity is:
You won’t always feel it. There’s no swelling or fever. But under the microscope? You’ll see overactive immune cells, stressed-out mitochondria, frayed signaling pathways, and cells responding to a thousand false alarms.
In short, the system that once protected you becomes your own internal heckler.
This is the biology of what scientists call inflammaging, a mashup of “inflammation” and “aging.” The term was coined to describe this precise phenomenon: the chronic, low-grade immune activation that creeps in with age, slowly disrupting systems that once worked in harmony.
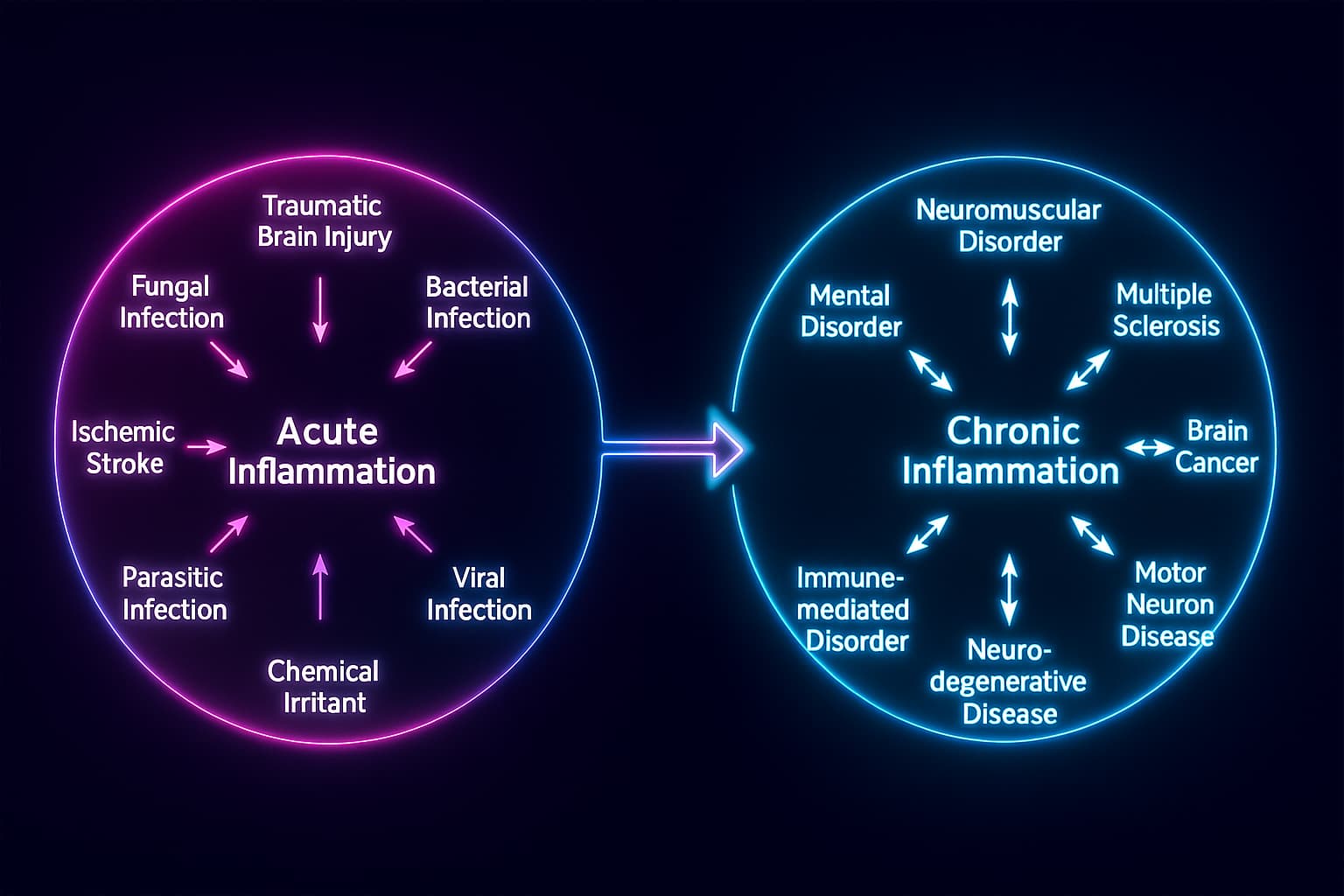
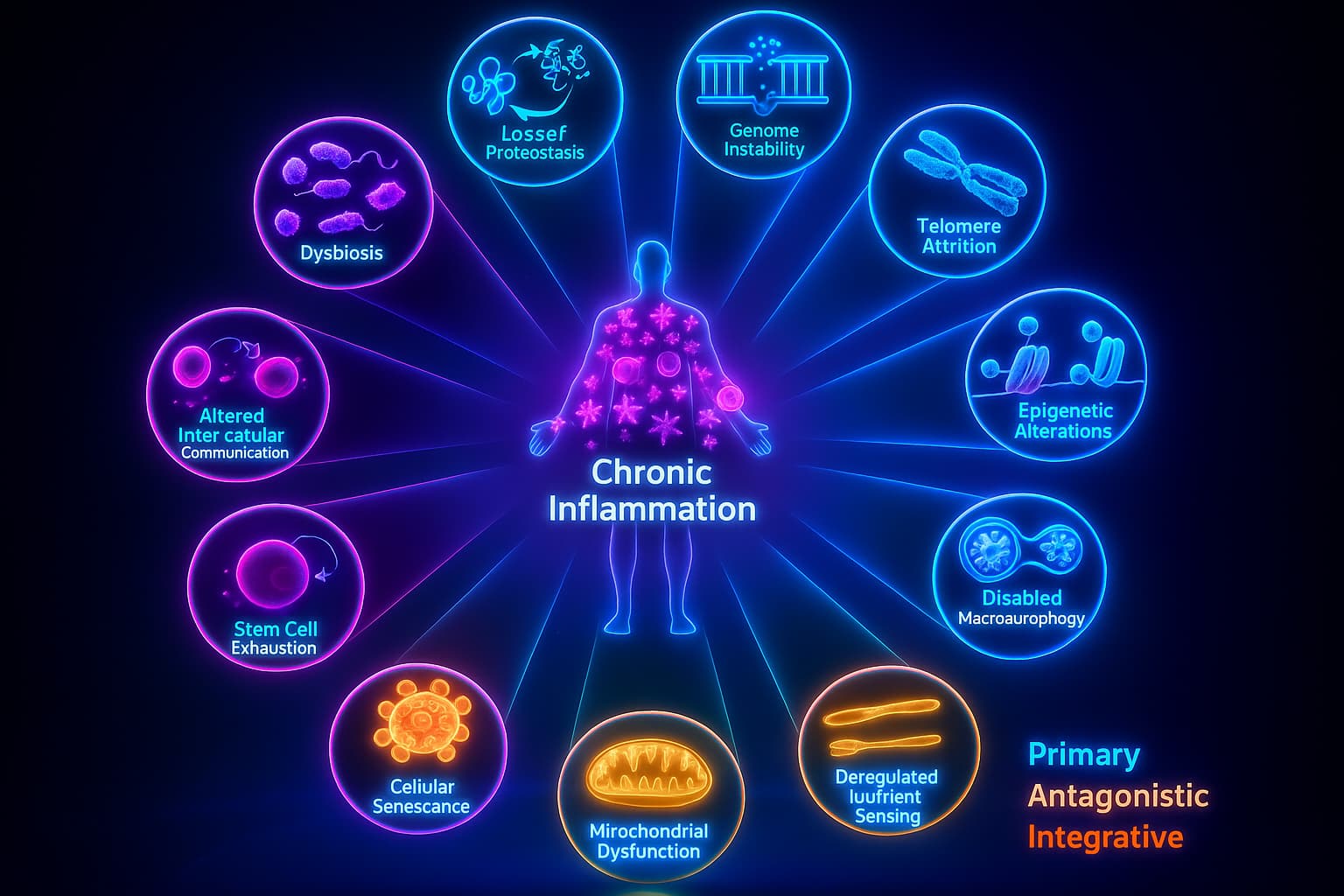
If you’ve been following our journey through the hallmarks of aging, you might notice something strange about this one. Chronic inflammation isn’t like the others. It’s not just a single process, it’s more like a consequence. A reaction. A biological echo of everything else that’s going wrong.
In fact, nearly every other hallmark feeds into it:
That’s why scientists see inflammaging not just as a standalone issue, but as a biological amplifier, a fire that catches when other systems fail, and then makes everything worse.
So let’s break down how this smoldering storm actually starts?
1. Cellular Trash Starts to Smell
Think of your cells as little cities with constant traffic: metabolism, repair, energy production. That creates waste. In youth, cleanup systems like autophagy and the proteasome act like janitors and garbage trucks, keeping everything tidy.
But with age, the cleanup slows down. Damaged proteins, broken organelles, and oxidized lipids start to pile up like uncollected trash – there is Loss of proteostasis. And just like a garbage bin left too long in the sun… it starts to stink.
The immune system picks up that “something’s wrong” signal and responds. Even though there’s no infection, the body acts like there is. triggering inflammation.
2. Senescent Cells Stir Up Trouble
Some cells stop dividing and go into retirement. That’s called cellular senescence and in theory, it’s a good thing. A way to prevent damaged cells from becoming cancerous.
But these cells don’t go gently into the night. They become grumpy neighbors who throw chemical tantrums, releasing inflammatory signals known as SASP (Senescence-Associated Secretory Phenotype).
These noisy secretions irritate surrounding tissues and spread inflammation. One senescent cell becomes ten. Ten become a toxic microenvironment.
This isn’t just aging, it’s age-related inflammation on a loop.
3. Mitochondria Cry Wolf
Mitochondria, your cell’s energy plants, are efficient in youth. But as they age, they get leaky. Their DNA breaks down, and they start releasing bits of themselves into the cell like distress flares, That’s called mitochondrial dysfunction.
To your immune system, this looks exactly like a viral invasion. It responds with firepower: cytokines, interferons, inflammation.
But the enemy isn’t real. It’s just a tired mitochondrion crying wolf. Over and over again.
4. The Gut Wall Starts to Crack
Your gut isn’t just a digestive tube, it’s a massive immune surveillance zone. A border wall between the outside world and your bloodstream.
As we age, that wall starts to break down. Microbes and toxins sneak through. This “leaky gut”, Also called dysbiosis, leaks microbial fragments into your blood and your immune system freaks out.
The result? Constant low-grade immune activation. The fire alarm blares all day. You might not feel it, but your immune system does.
And that’s inflammaging, burning from the inside out.
5. DNA Damage Triggers the Alarm
As cells replicate, DNA takes damage from oxidation, UV light, replication errors, telomere shortening or just time. Usually, this gets repaired. But aging slows the repair crews.
When DNA breaks build up, your cells flip on emergency systems like cGAS-STING, which normally defend against viruses. But now? They’re just reacting to your own broken blueprints.
It’s like mistaking static on the radio for an incoming missile. The immune system gears up, fires off cytokines, and inflammation spreads.
6. The Recycling Systems Break Down
Processes like autophagy are supposed to break down damaged cell parts and recycle them. But with age, autophagy slows. Proteins misfold, mitochondria malfunction, lipids oxidize and the cell becomes cluttered.
The result? Cellular stress. And with it, inflammatory pathways like NF-κB turn on, not because of infection, but because the cell is drowning in junk.
Your body interprets this mess as danger. And danger means inflammation.
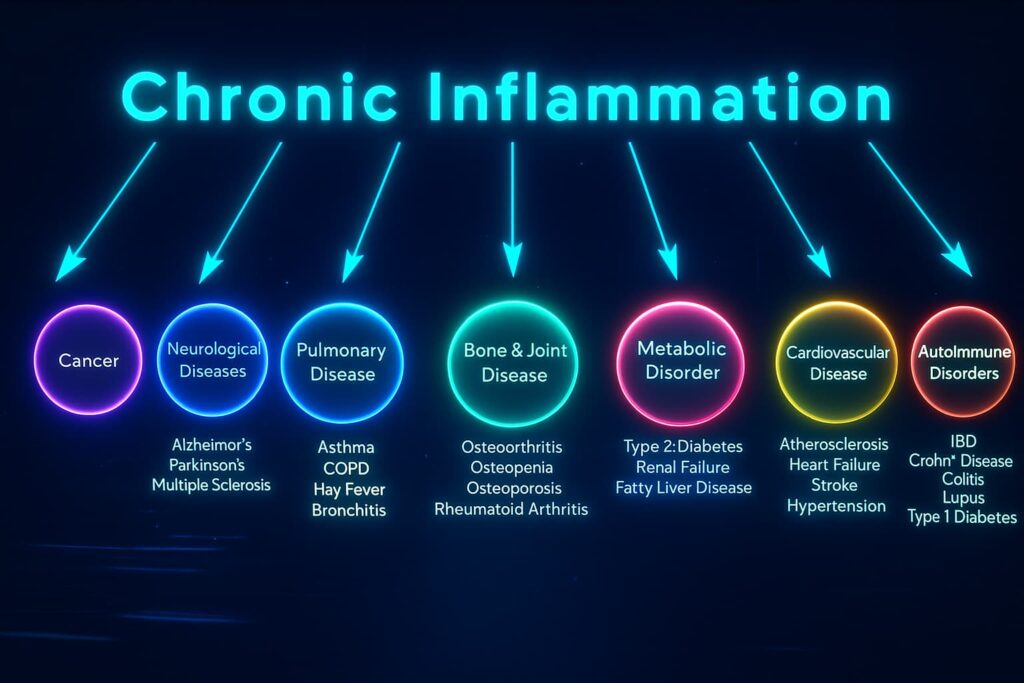
By now, you know that chronic inflammation is the slow burn behind the scenes fueled by cellular trash, damaged DNA, leaky mitochondria, and more. But what happens when that fire keeps burning?
Well… everything.
Because this isn’t just an immune system problem. It’s a whole-body malfunction, one that touches nearly every organ, every function, and every one of the hallmarks of aging.
Let’s take a tour through the damage
1. The Heart Loses Its Rhythm
Chronic inflammation and aging go hand-in-hand in your cardiovascular system. Inflammatory molecules like IL-6 and TNF-α irritate blood vessel linings, promoting plaque buildup and hardening of the arteries (aka atherosclerosis).
This makes your heart work harder, raises your blood pressure, and sets the stage for heart attacks and strokes, some of the leading causes of age-related mortality.
The result? Inflammation and longevity don’t mix well here. The longer the fire burns, the more damage it does to your most vital engine.
2. The Brain Slows and Stiffens
Your brain thrives on clean signals and smooth coordination. But inflammaging turns up the static.
Microglia (the brain’s immune cells) go from helpful janitors to overreactive gossipers. Instead of cleaning up messes, they start attacking healthy neurons. Meanwhile, the blood-brain barrier weakens, letting inflammatory molecules from the body sneak into the brain.
Over time, this neuroinflammation contributes to cognitive decline, memory loss, and the progression of diseases like Alzheimer’s and Parkinson’s.
This is age-related inflammation at its most personal turning thought, clarity, and identity into collateral damage.
3. Cells Stop Listening
Chronic inflammation scrambles the cellular conversation. Receptors become desensitized, signaling pathways become overloaded, and hormones like insulin and leptin stop working properly.
This contributes to:
And when these signals break down, the body spirals into metabolic chaos. Inflammaging isn’t just noisy, it drowns out the important messages.
4. Stem Cells Go Silent
Stem cells are your repair crew: rebuilding tissues, replenishing blood, restoring the skin. But chronic inflammation makes them retreat. It shortens their lifespan, exhausts their potential, and even pushes them toward senescence.
The result? Slower healing, thinner skin, weaker muscles, and brittle bones. You’re not just aging, you’re running out of replacements, a process known as stem cell exhaustion.
This is why inflammation and longevity are so tightly linked: if your stem cells can’t function, your body loses the ability to bounce back.
5. Tissues Break Down
Every part of your body relies on a delicate balance between damage and repair. But with inflammaging dominating the landscape, that balance shifts.
Joints become swollen and painful. Muscle fibers weaken. Skin loses its elasticity. The extracellular matrix (the scaffold between cells) becomes stiff and scarred.
This isn’t just wear and tear. It’s age-related inflammation reshaping your tissues from the inside out.
6. The Immune System Turns on Itself
In youth, your immune system is sharp, coordinated, and precise. But chronic inflammation makes it overactive and confused. Some cells overreact. Others miss threats entirely.
This leads to:
In other words, inflammation isn’t just the immune system in action, it’s the immune system off script.
The Takeaway: A Fire That Spreads Everywhere
The story of chronic inflammation and aging isn’t about one broken part, it’s about a whole-body system stuck in the wrong gear.
From brain fog to heart disease, from diabetes to frailty, inflammaging shows up in subtle, persistent ways. slowly but powerfully eroding the body’s resilience.
It makes every other hallmark of aging worse. It’s not the cause of aging, it’s the accelerant.
And the good news? Fires can be cooled. Signals can be calmed. And aging can be slowed.
In the next section, we’ll explore exactly how to quench the flames of chronic inflammation from lifestyle shifts to science-backed supplements.
Chronic inflammation and aging may feel inevitable, but the truth is: your body listens closely to what you do every day. From what you eat to how you move, these choices send biochemical messages that either fuel inflammaging, or calm it down.
When it comes to fighting chronic inflammation and aging, your daily habits are the strongest medicine.
1. Eat to Soothe, Not Inflame
Certain foods calm inflammation. Others pour fuel on the fire.
Avoid ultra-processed foods, sugar, and refined oils, they spike blood sugar, irritate the gut, and trigger inflammaging.
2. Move to Reset Immunity
Exercise triggers a mini stress, but it teaches your body how to bounce back stronger.
It clears damaged cells, boosts insulin sensitivity, and reduces age-related inflammation, all while helping you sleep better and feel sharper.
3. Sleep Deeply
Sleep is your nightly anti-inflammatory reboot.
Without it, NF-κB and TNF-α stay switched on, driving inflammation and immune confusion. With it? Your body resets, repairs, and rewires.
4. Stress Less
Stress is a silent source of chronic inflammation.
Breathwork, meditation, and social connection lower cortisol and help the nervous system shift from “fight or flight” to “rest and repair.”
Certain compounds can reinforce your anti-inflammatory lifestyle and target the biological machinery driving inflammaging.
1. Omega-3s (EPA & DHA)
These fatty acids are converted into resolvins, which help end inflammatory cycles and repair tissues. They also lower CRP (C-reactive protein), a key marker of age-related inflammation linked to heart disease and cognitive decline.
2. Fisetin & Quercetin
Found in plants like strawberries and onions, these senolytics help eliminate senescent cells, aging cells that flood tissues with inflammatory SASP molecules. Clearing them reduces chronic inflammation at the source.
3. Curcumin
This turmeric compound blocks NF-κB and COX-2, two major inflammatory drivers. Think of it as turning down the volume on your immune system’s alarm system.
4. Resveratrol
Famous from red wine, resveratrol reduces oxidative stress and activates SIRT1, a gene that helps cells repair and stay resilient under pressure. It supports both inflammation control and longevity.
5. Probiotics + Prebiotics
Your gut is a major inflammation hub.
The result? A stronger gut barrier, less immune overreaction, and reduced chronic inflammation and aging signals traveling through the bloodstream.
Not medical advice. Always check with your doctor before using any supplement.
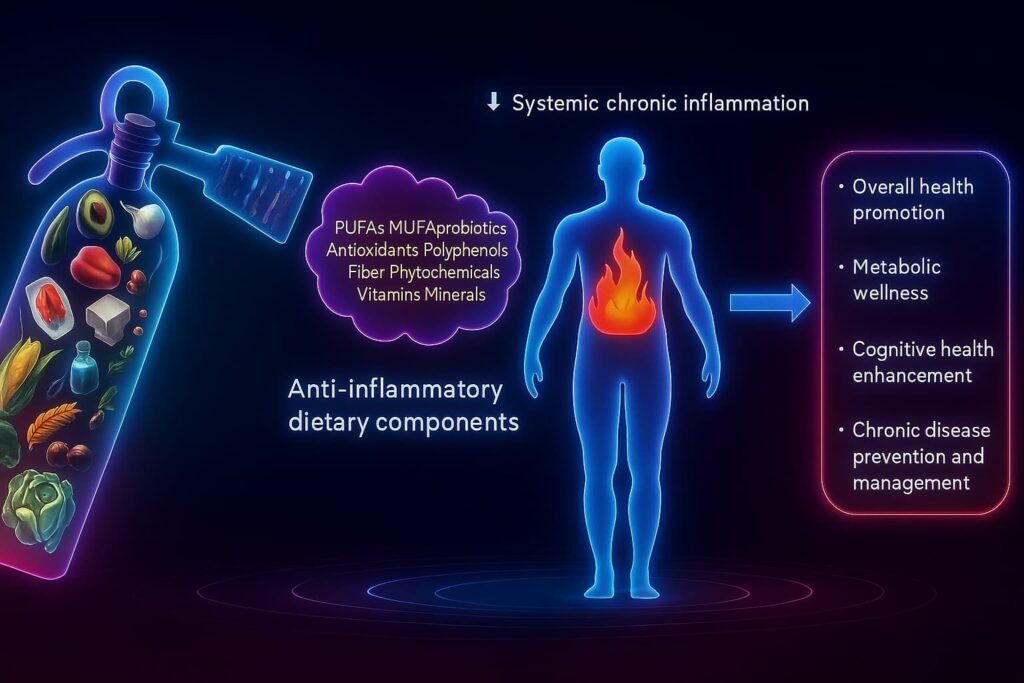
Lifestyle and supplements can cool chronic inflammation but what if we could go deeper? Instead of just managing inflammaging, what if we could reprogram it at the source?
That’s exactly where science is headed.
1. Senolytics: Clearing the Cellular Smoke
Senescent cells (the “zombie” cells that won’t die) are a major source of age-related inflammation through their toxic secretions (SASP).
Senolytics like fisetin, dasatinib, and navitoclax target and remove these cells, helping:
These therapies directly reduce inflammaging, and early human trials look promising.
2. Exosome Therapy: Delivering the Right Messages
Cells communicate through tiny packages called exosomes, like molecular mail.
As we age, these messages grow chaotic. But scientists are developing engineered exosomes that carry anti-inflammatory instructions, helping to:
It’s still experimental, but it may become a powerful way to retrain the immune system from the inside out.
3. Gene Therapy: Rewriting Inflammation Itself
The most radical approach? Editing the genes that control inflammation.
Some therapies aim to dial down NF-κB, the main genetic switch for inflammatory signals. Others boost longevity genes like FOXO3 and SIRT1, which help cells resist stress and stay youthful.
The goal isn’t just to reduce damage, it’s to rewire how we age.
These future tools are still in development, but the direction is clear:
Chronic inflammation and aging won’t just be treated, they’ll be reprogrammed.
Chronic inflammation and aging are more connected than we ever imagined. As we age, our immune system doesn’t just slow down, it stays stuck in overdrive. This constant, low-grade immune activation is known as inflammaging, and it quietly fuels nearly every type of decline we associate with getting older.
From senescent cells releasing inflammatory noise, to mitochondria sending false alarms, to a leaky gut dripping confusion into the bloodstream, chronic inflammation becomes the biological background hum that damages tissue, scrambles communication, and wears down resilience.
This invisible fire:
But this isn’t irreversible.
With smart, daily choices, anti-inflammatory nutrition, consistent movement, restful sleep, and stress reduction, you can turn down the flame. Add in targeted supplements like omega-3s, curcumin, quercetin, and probiotics, and you’re giving your cells the tools to cool the fire from the inside out.
And the future? Even brighter. Emerging therapies like senolytics, exosome therapy, and gene editing are paving the way to not just calm inflammation but to reprogram aging itself.
Because when we quiet chronic inflammation, we don’t just feel better.
We age better.
And that means a longer, clearer, more vibrant life, from the inside out
Now that you’ve unlocked the science behind Chronic inflammation and aging, why stop here? The aging process is a mosaic and Chronic inflammation just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?