Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“As we age, the harmony of our gut orchestra fades and with it, the symphony of health falters.“
Welcome, caretaker of the inner garden.
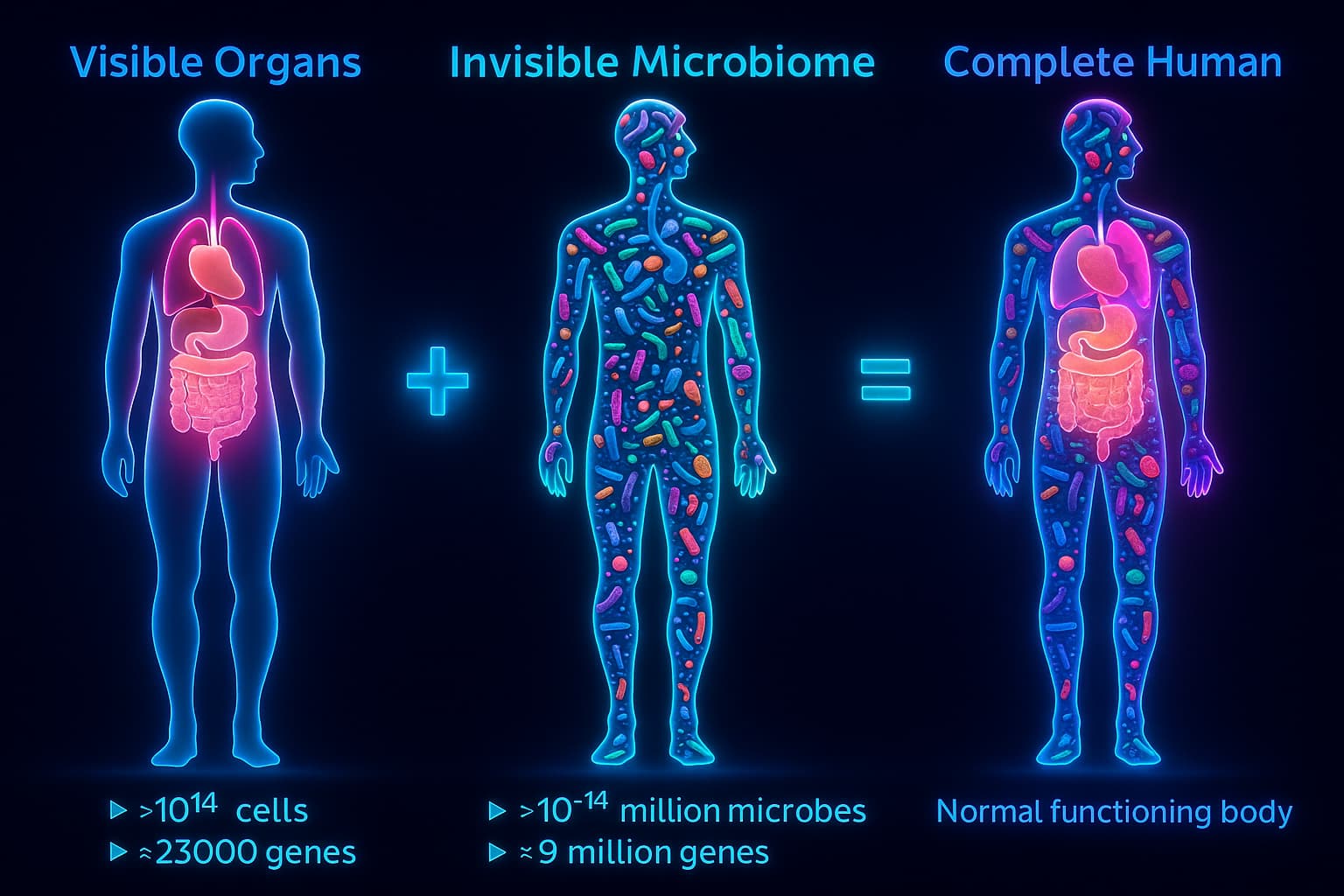
In exploring the hallmarks of aging, we often look to DNA, mitochondria, and cellular repair systems. Yet one of the most underappreciated forces shaping energy, immunity, and even mood lies within your gut. Here lives a vast and intricate ecosystem, the gut microbiome, trillions of microbes working in harmony to digest food, regulate the immune system, produce vital compounds, and even send signals to the brain.
In youth, this microbial garden is lush and diverse. Beneficial strains flourish, balance prevails, and inflammation is kept in check. With age, that balance begins to falter. Diversity fades, helpful species dwindle, and harmful microbes move in. This shift, known as dysbiosis, is now recognized as a microbial imbalance hallmark of aging.
The link between dysbiosis and aging runs deep. As the microbiome deteriorates, it fuels systemic inflammation, weakens nutrient absorption, confuses immune defenses, and even disrupts brain function. These changes, in turn, accelerate other hallmarks of aging, creating a cycle where aging and gut decline feed into each other.
In this exploration, we will uncover:
To understand gut microbiome and longevity is to realize that nurturing this inner ecosystem can strengthen the foundations of health itself. When the microbiome thrives, so does the body’s resilience, from mitochondria to mood.
Let us step into this hidden garden and explore how to help it bloom again.
Your gut is not just a digestive tube, it’s a bustling metropolis teeming with life. Trillions of microbes (mostly bacteria, but also fungi, viruses, and archaea) make up your gut microbiome, a microscopic ecosystem that supports everything from nutrient absorption and hormone production to immune regulation and brain function.
In youth, this ecosystem is diverse, cooperative, and anti-inflammatory, a symphony of microbial teamwork. But that harmony is fragile.
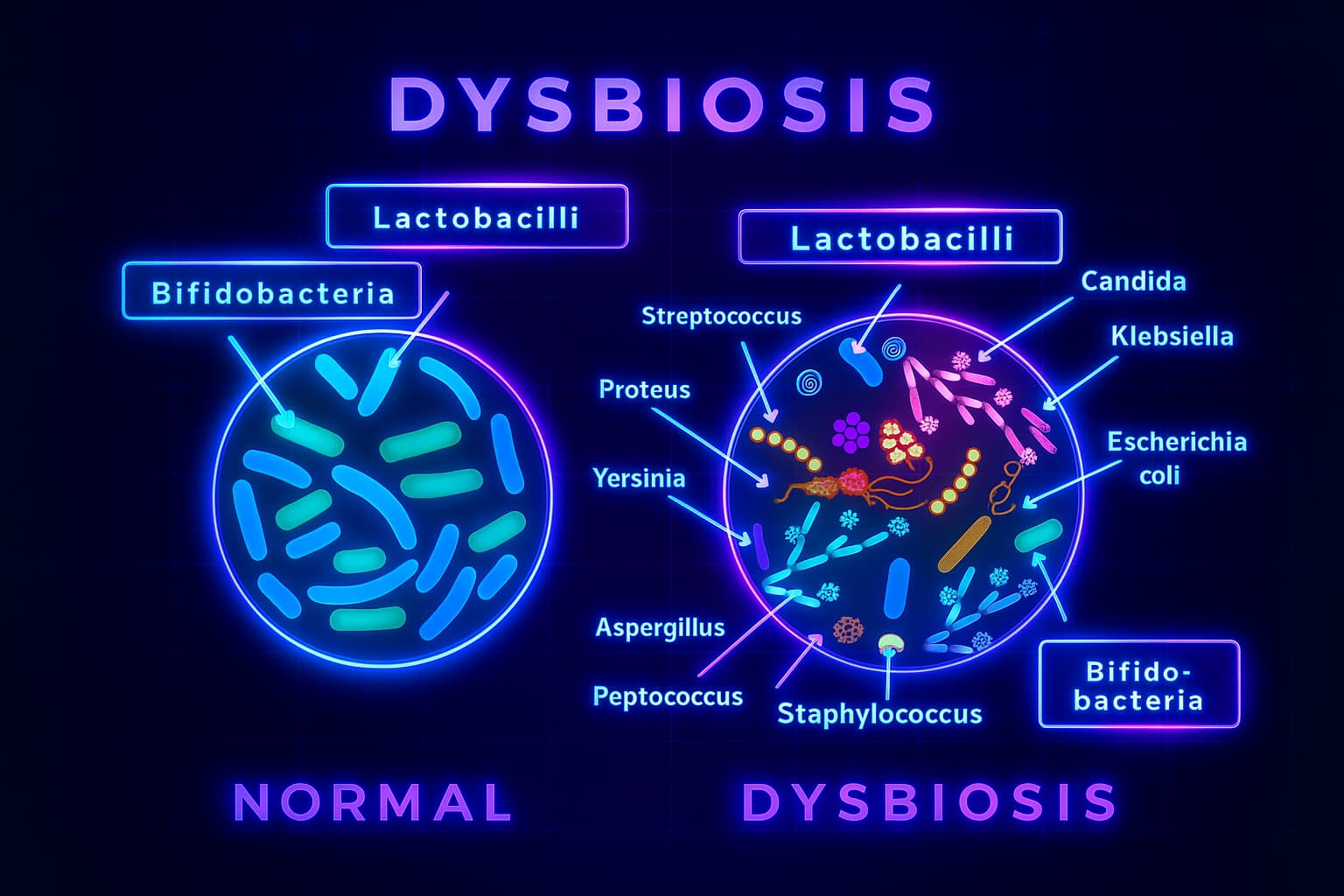
Dysbiosis is what happens when the balance shifts. The helpful species decline, and the troublemakers, often inflammatory, sugar-loving, or immune-disrupting strains take over. It’s like your lush inner garden has been overrun with weeds. The soil quality drops, nutrient production falters, and chronic inflammation takes root.
What a healthy gut microbiome normally does?
But with dysbiosis and aging, that microbial harmony falls apart. Inflammation spikes. Gut permeability increases (hello, leaky gut). The immune system starts to attack shadows. Nutrient absorption falters. Cognitive clarity dims. Energy dips.
So the gut microbiome and longevity aren’t just linked, they’re inseparable.
And the microbiome doesn’t just influence how you digest… it shapes how you think.
That flutter in your stomach before a big decision? It’s not just nerves, it’s your second brain talking. That’s what we mean by a gut feeling.
This isn’t just a digestive issue, it’s a whole-body concern. Dysbiosis is now recognized as a microbial imbalance hallmark of aging, a foundational process that speeds up decline across multiple systems.
Next, let’s dig into why this microbial shift happens with age and how it drives the deeper currents of aging and gut health decline.
Your gut microbiome is like a rainforest: wild, diverse, and full of species playing unique roles in keeping the ecosystem thriving. But as we age, the canopy thins, the soil erodes, and the balance tips. Welcome to dysbiosis and aging, where the once-vibrant garden of your gut slowly loses its harmony.
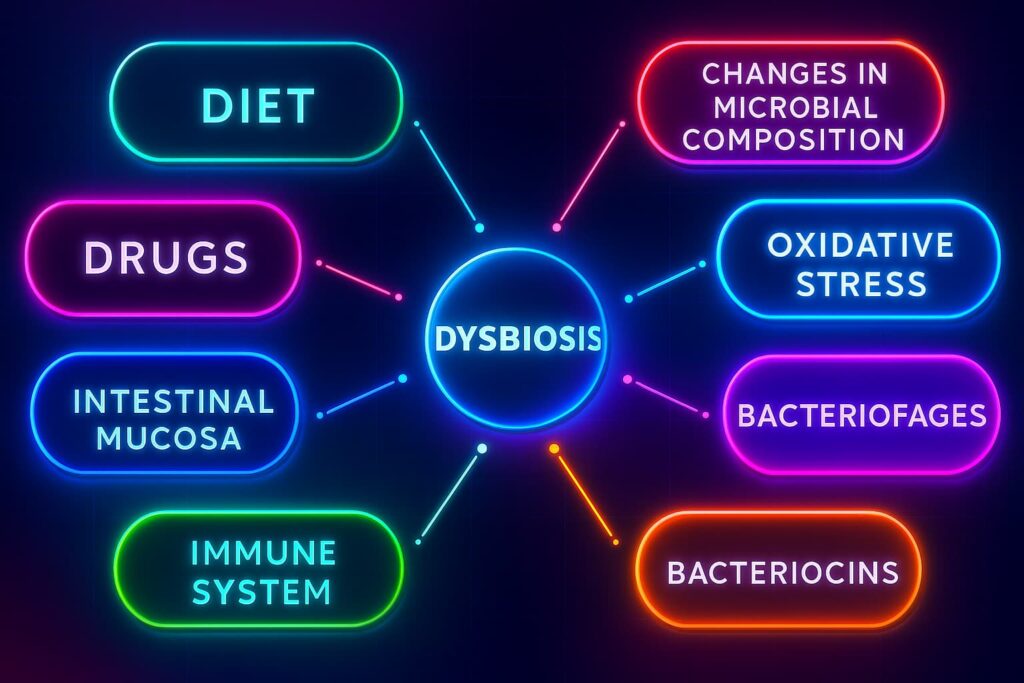
So what actually causes this microbial mess?
1. The Good Guys Pack Up and Leave
Some of your most beneficial microbes like Faecalibacterium and Akkermansia are like expert landscapers. They fertilize the soil, reduce inflammation, and help patch up damage.
But with age, the gut becomes a tougher neighborhood. Mucus thins, nutrients change, and inflammation rises, driving these peacekeepers out. Without them, weeds (a.k.a. inflammatory bacteria) take over. And this shift? It’s a classic sign of microbial imbalance as a hallmark of aging.
2. The Gut Wall Becomes a Leaky Fence
Your mucus layer is like a velvet rope, it keeps microbes in their lane. But aging tears holes in that barrier. Now microbes sneak past and ring alarms, waking up the immune system like it’s under attack.
This is how dysbiosis and longevity collide: inflammation rises, and your gut starts sending panic signals to the rest of the body including your brain.
These misfired signals are a hallmark of altered intercellular communication, creating ripple effects in tissues far beyond the gut even contributing to neuroinflammation and mitochondrial dysfunction in the brain.
3. The Immune System Forgets Who the Friends Are
Your gut’s immune system is supposed to play favorites, tolerating friendly bacteria while targeting invaders. But with age, it gets confused.
Helpful microbes get flagged as threats. Peace turns to war. The result? A vicious loop of inflammatory dysbiosis, where even good guys get caught in the crossfire.
4. The Chemistry Shifts
Young microbes produce calming molecules like butyrate, kind of like compost for your gut. But aging changes the mix. Now you get more acidic, pro-inflammatory byproducts and fewer soothing ones.
This tilts the balance toward microbes that thrive in chaos. It’s not just unpleasant, it’s one of the ways gut microbiome and longevity drift apart with age.
5. Yes, Microbes Age Too
Believe it or not, some bacteria show signs of aging: DNA damage, stress sensitivity, and loss of function. They’re like tired gardeners who forget to prune the hedges or water the soil.
Just like your cells, aging microbes experience genomic instability, loss of proteostasis, and even epigenetic drift, reminding us that aging isn’t limited to human cells alone.
Bottom line?
Your microbiome isn’t just collateral damage in aging, it’s part of the control system. And when this network frays, the consequences ripple out across your entire body.
So no, the microbiome doesn’t just affect digestion.
It affects how you think, feel, heal, and age.
Now that’s what you call a gut feeling.
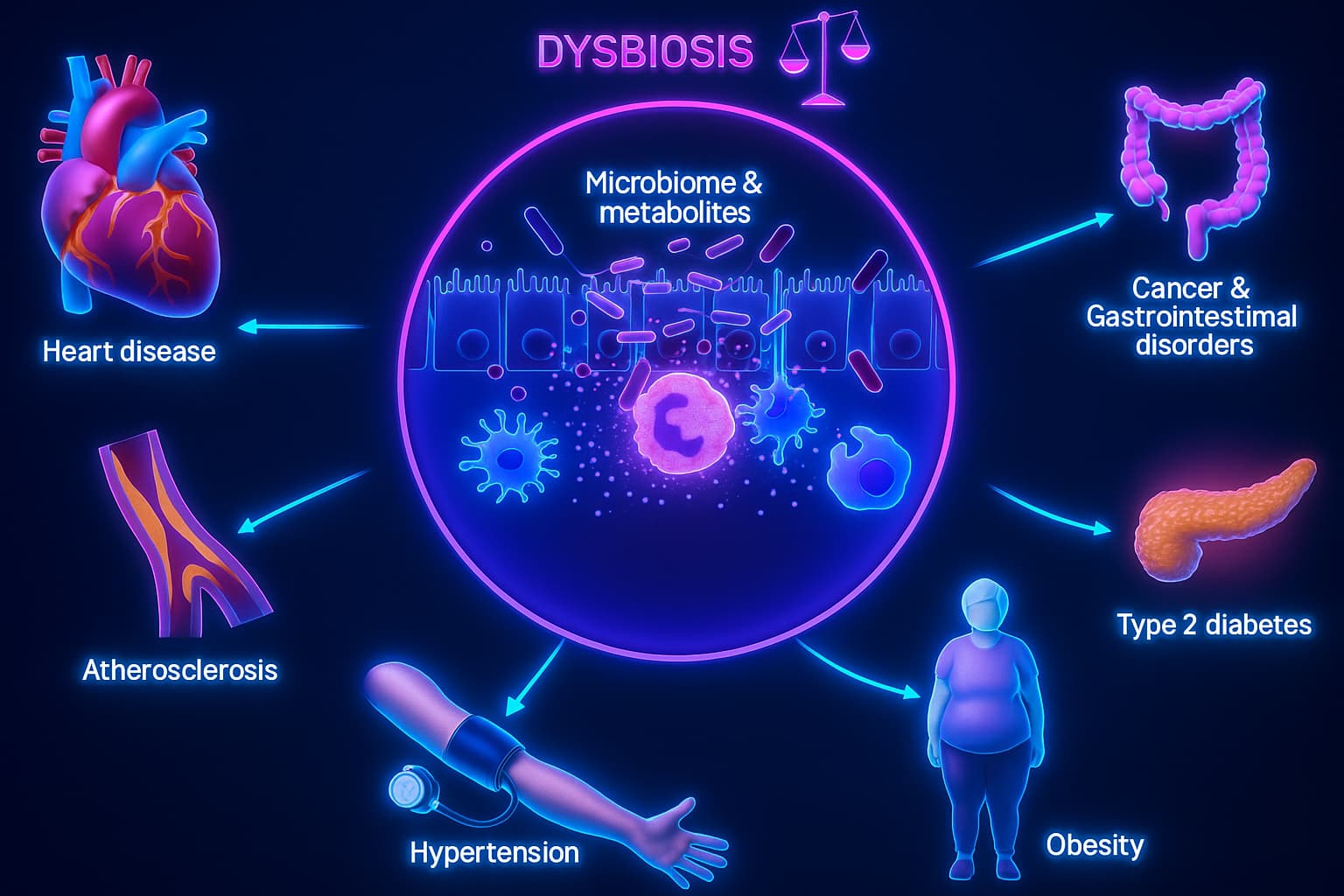
Dysbiosis isn’t just something happening in the shadows of your intestines. It shows up loudly in how you feel, heal, think, and age. This isn’t just a tummy ache. It’s a full-blown biological cascade that starts in your gut and spreads through your entire network.
Let’s trace the ripples:
1. Bloating, Gas, and Digestive Drama
Let’s start with the obvious. When the gut microbiome falls out of balance, your digestion goes haywire. You might feel like a balloon animal, thanks to bacteria that love to ferment sugars into gas.
The missing species? They’re the ones that usually break down fiber into anti-inflammatory short-chain fatty acids. Without them, your gut lining weakens, inflammation rises, and everything gets… louder.
2. Systemic Inflammation
This is where dysbiosis stops being “just a gut problem.” Harmful microbes start producing endotoxins, your gut wall gets leaky, and microbial bits spill into the bloodstream.
The immune system freaks out. Cytokines flood in. You’ve now triggered inflammatory aging, a key pathway linking dysbiosis and longevity decline.
This low-grade, body-wide inflammation? It’s like static noise on every cellular channel: disrupting metabolism, brain health, and tissue repair.
3. Brain Fog, Anxiety, and Mood Swings
Ever heard of the gut-brain axis? It’s real, a direct line of communication between your microbes and your mind. A healthy gut microbiome produces mood-regulating neurotransmitters like serotonin and GABA.
But with dysbiosis and aging, these messages get scrambled. You may feel foggy, anxious, or “off,” even if everything else looks normal. This is the microbiome whispering or yelling to your nervous system.
So no, you’re not imagining it. Gut health decline in aging really can cloud your thoughts.
4. Immune Confusion
About 70% of your immune system lives in the gut. When the microbiome is thriving, it trains your immune cells to be smart, focused, and calm.
But microbial imbalance disrupts this training. Now your immune system becomes overactive or sluggish, missing threats, attacking friendly tissues, and losing resilience. Think autoimmunity, allergies, or getting sick more often than you used to.
This is why dysbiosis is now recognized as a hallmark of aging, it weakens one of your body’s most essential defense systems.
5. Nutrient Deficiencies & Fatigue
Certain gut bacteria help extract and synthesize key nutrients like B vitamins, vitamin K, magnesium, and amino acids.
But when dysbiosis sets in, nutrient absorption falters. You might eat well and still feel tired, weak, or slow to recover. It’s not your willpower, it’s the microbes behind the curtain.
6. Weight Gain and Metabolic Confusion
Some bacteria love sugar and when they dominate, your cravings spike. Others help regulate insulin sensitivity and fat storage.
With dysbiosis and aging, this balance unravels. You’re more likely to gain visceral fat, experience insulin resistance, and develop metabolic syndrome, These changes are tightly linked to deregulated nutrient sensing, a hallmark of aging that affects insulin pathways, AMPK activity, and mTOR signaling, all crucial to maintaining metabolic health and longevity.
In Short:
Your microbes are your metabolic managers, immune coaches, and mood messengers.
And when dysbiosis tips the balance, every system from brain to belly starts to feel the strain.
But the good news is that this is one hallmark of aging we can actually grow our way out of.
Even though dysbiosis and aging tend to go hand-in-hand, your gut microbiome is surprisingly resilient. Treat it right, and it responds fast, shifting back toward balance, reducing inflammation, and restoring that crucial connection between gut health and longevity.
Let’s explore how.
1. Eat More Prebiotics
Prebiotics are fibers your human cells can’t digest, but your microbes love them. Foods like garlic, leeks, asparagus, oats, and green bananas feed beneficial bacteria like Bifidobacteria and Akkermansia. As they ferment these fibers, they release short-chain fatty acids (like butyrate and propionate), which strengthen the gut lining, suppress inflammation, and regulate immune responses.
This not only helps repair leaky gut but also restores the microbial diversity that erodes with age, directly countering the microbial imbalance hallmark of aging.
2. Reseed with Probiotic-Rich Foods
Fermented foods like kimchi, kefir, sauerkraut, and miso provide live cultures that increase microbial variety. These helpful bacteria compete with pathogenic strains for space and nutrients, reducing dysbiosis and calming the immune system.
With aging, the natural diversity of the microbiome shrinks. Reintroducing good bugs helps restore that lost complexity, enhancing the gut microbiome and longevity connection.
3. Reduce Sugar and Ultra-Processed Foods
Refined sugars and additives feed pro-inflammatory bacteria and fungi like Candida, which thrive in dysbiotic environments. These “bad actors” produce toxins (endotoxins, for instance) that disrupt the gut barrier and trigger systemic inflammation.
By starving these strains, you reduce microbial noise and create space for beneficial species to regrow, easing the gut health decline linked to aging.
4. Move Your Body
Exercise increases microbial diversity, especially in butyrate-producing species. It also boosts intestinal transit time, keeping pathogens from lingering and overgrowing.
Studies show active individuals have more resilient, anti-inflammatory microbiomes, even into old age. This makes movement a core part of reversing dysbiosis and aging.
5. Prioritize Deep Sleep
Your gut bacteria follow a circadian rhythm, just like you. Poor sleep disrupts this microbial clock, leading to lower diversity and increased gut permeability.
Good sleep enhances mucosal immunity, promotes SCFA production, and keeps communication between your brain and gut clear, key for slowing the cascade of dysbiosis and longevity loss.
Sometimes your gut needs more than a good meal, it needs strategic allies. These targeted supplements work behind the scenes to rebuild microbial harmony, strengthen the gut barrier, and cool down inflammation.
1. Prebiotic Fibers (Inulin, PHGG, FOS)
Think of these as gourmet meals for your good bacteria. Prebiotics like inulin and partially hydrolyzed guar gum (PHGG) resist digestion in the upper gut and arrive intact in the colon, where they’re fermented into short-chain fatty acids (SCFAs) like butyrate. Butyrate not only nourishes colon cells, it also reduces NF-κB activation (a key inflammatory pathway), supports immune tolerance, and improves tight junction integrity to prevent “leaky gut.” With age, these SCFA levels decline but feeding the right bugs brings the balance back.
2. Spore-Based Probiotics (e.g. Bacillus coagulans)
Unlike traditional strains that often die in the stomach, spore-formers have tough protein coats that let them survive gastric acid and reach the intestines intact. Once there, they germinate and help push out pro-inflammatory pathogens, increase microbial diversity, and modulate local immune activity. These are especially helpful after antibiotics or during periods of stress, when dysbiosis and aging tend to spiral together.
3. Psychobiotics (e.g. Lactobacillus rhamnosus, Bifidobacterium longum)
These specialized strains produce neuroactive compounds like GABA, serotonin, and even dopamine precursors. Through the vagus nerve and local immune signaling, they influence the gut-brain axis, helping regulate mood, reduce anxiety, and even improve memory. It’s a wild but real connection: your gut microbiome and longevity are tied to how you feel and think, not just how you digest.
4. L-Glutamine
This amino acid is a favorite fuel for intestinal epithelial cells. It supports mucosal repair, tight junction assembly, and the production of secretory IgA, your gut’s first immune defense. When microbial imbalance damages the gut lining, glutamine helps “seal the leaks” and calm inflammation.
5. Polyphenols (Curcumin, Green Tea Extract, Resveratrol)
More than just antioxidants, polyphenols act like ecosystem engineers. They inhibit pro-inflammatory species like E. coli and Clostridium difficile while encouraging the growth of friendly butyrate-producing microbes. Plus, many polyphenols enhance barrier function and reduce circulating endotoxins (like LPS), which spike in aging and gut health decline.
6. Tributyrin (Butyrate precursor)
Butyrate is a cornerstone of gut longevity, feeding colonocytes, boosting Treg immune cells, and suppressing inflammatory pathways. Tributyrin is a time-release version that survives digestion and releases butyrate in the lower gut, where it’s most needed. Low butyrate is a biomarker of both dysbiosis and aging and restoring it can help calm the immune system and revive the gut-brain connection.
Together, these lifestyle and supplement strategies help reverse gut dysbiosis, restore healthy microbial communication, and support your body’s most powerful internal ecosystem. Because when the gut is thriving everything else, from mood to metabolism gets back on track.
Next up? Let’s zoom in on the cutting-edge therapies that might reengineer your gut flora from the inside out.
Not medical advice. Always check with your doctor before using any supplement.
While lifestyle and supplements help your gut bloom, science is digging even deeper, developing therapies that rebuild the microbiome from the inside out. These aren’t just garden tools… they’re microbial power tools.
Fecal Microbiota Transplantation (FMT)
Transferring gut microbes from healthy young donors into older individuals has shown promise in animal studies: restoring cognition, reducing inflammation, and improving metabolism. Think of it as reseeding depleted soil with rich, fertile compost. It’s an emerging strategy to fight dysbiosis and aging head-on.
Precision Microbiome Editing
Researchers are learning how to edit gut microbes like code, dialing down inflammation-promoting strains and boosting longevity allies like Faecalibacterium prausnitzii. It’s the future of gut microbiome and longevity, one gene switch at a time.
Postbiotics
No bacteria, just their beneficial byproducts. Postbiotics: like butyrate, peptides, or microbial messengers offer the benefits of a healthy microbiome without the need for live bugs. Especially helpful when microbial imbalance in aging makes standard probiotics risky.
Gut-Brain Axis Therapies
New treatments are targeting the biochemical superhighway between your gut and brain, aiming to reduce neuroinflammation and enhance mood by restoring healthy gut signaling. Proof that aging and gut health decline can affect far more than digestion.
Aging doesn’t happen in isolation, it’s a symphony of breakdowns, and your gut microbiome is both a conductor and a casualty.
As we’ve seen, dysbiosis and aging go hand in hand. When the gut microbiome falls out of balance, it doesn’t just cause bloating or discomfort, it sparks a chain reaction of inflammation, immune confusion, nutrient loss, and even cognitive decline. This microbial imbalance hallmark of aging accelerates deterioration across every system, from your brain to your bones.
With the right foods, lifestyle habits, and science-backed supplements, you can regrow a thriving gut ecosystem, one that supports energy, sharp thinking, emotional resilience, and immune balance. You can shift the tide from breakdown to regeneration.
And with emerging therapies on the horizon, from precision microbiome editing to postbiotic medicine, we’re entering an era where gut microbiome and longevity may be as treatable as cholesterol or blood pressure.
Because the real root of longevity? It just might be in your roots, the trillions of tiny organisms in your gut.
Nurture them, and they’ll return the favor, one healthy year at a time.
Now that you’ve unlocked the science behind Dysbiosis and aging, why stop here? The aging process is a mosaic and dysbiosis is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?