Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“You are as young as your stem cells are active“
Welcome, guardian of the body’s renewal.
If you have been following this journey through the hallmarks of aging, you know aging is not simply about surface changes like wrinkles or slower movement. It is about the quiet breakdown of systems that once kept you strong and resilient. At the heart of this is stem cell exhaustion, one of the most fundamental and underappreciated drivers of aging.
The connection between stem cell exhaustion and aging runs deep. Stem cells are your body’s internal repair crew, responsible for replenishing tissues, healing injuries, and replacing worn-out cells. They keep your blood healthy, your skin renewed, your gut lining intact, and your immune system ready to defend you. But as years pass, this regenerative force dwindles. Some stem cells disappear entirely, while others slip into dormancy, no longer dividing or responding to the body’s needs.
Stem cell exhaustion is considered an integrative hallmark of aging, meaning it often appears after other types of cellular damage, such as DNA breaks, epigenetic changes, and metabolic imbalances, have taken their toll. When the repair crew fails, it signals that the deeper forces of aging have firmly taken hold.
In this exploration, we will uncover:
To understand stem cell exhaustion and aging is to see the difference between a body that can repair itself and one that cannot. Protecting this repair network may be one of the most powerful ways to extend both lifespan and vitality.
Let us step inside the body’s renewal system and explore how to keep it alive and thriving.
Stem cells are the body’s master builders, cells that remain in a flexible, non-final state, like clay that hasn’t yet been molded. Unlike fully developed cells that are committed to a single role, stem cells retain the unique ability to become many different cell types. This flexibility is central to their role in tissue regeneration, cellular repair, and long-term health.
The Stem Cell Niche (Quiet Reserve)
Most stem cells live quietly in protective microenvironments called niches. These niches are nestled in organs like the bone marrow, intestines, muscles, and skin. The niche acts like a dormant training ground, a calm place where stem cells rest, receiving biochemical signals that keep them healthy, stable, and ready to respond.
When the body experiences stress, injury, or cellular loss, the niche “wakes up” its residents. Stem cells then jump into action, dividing to rebuild what’s been damaged.
Division With Purpose: Self-Renewal and Repair
When activated, a stem cell performs a carefully balanced division:
This elegant system keeps the body’s tissues youthful and responsive, especially in fast-renewing areas like the skin, gut, and blood.
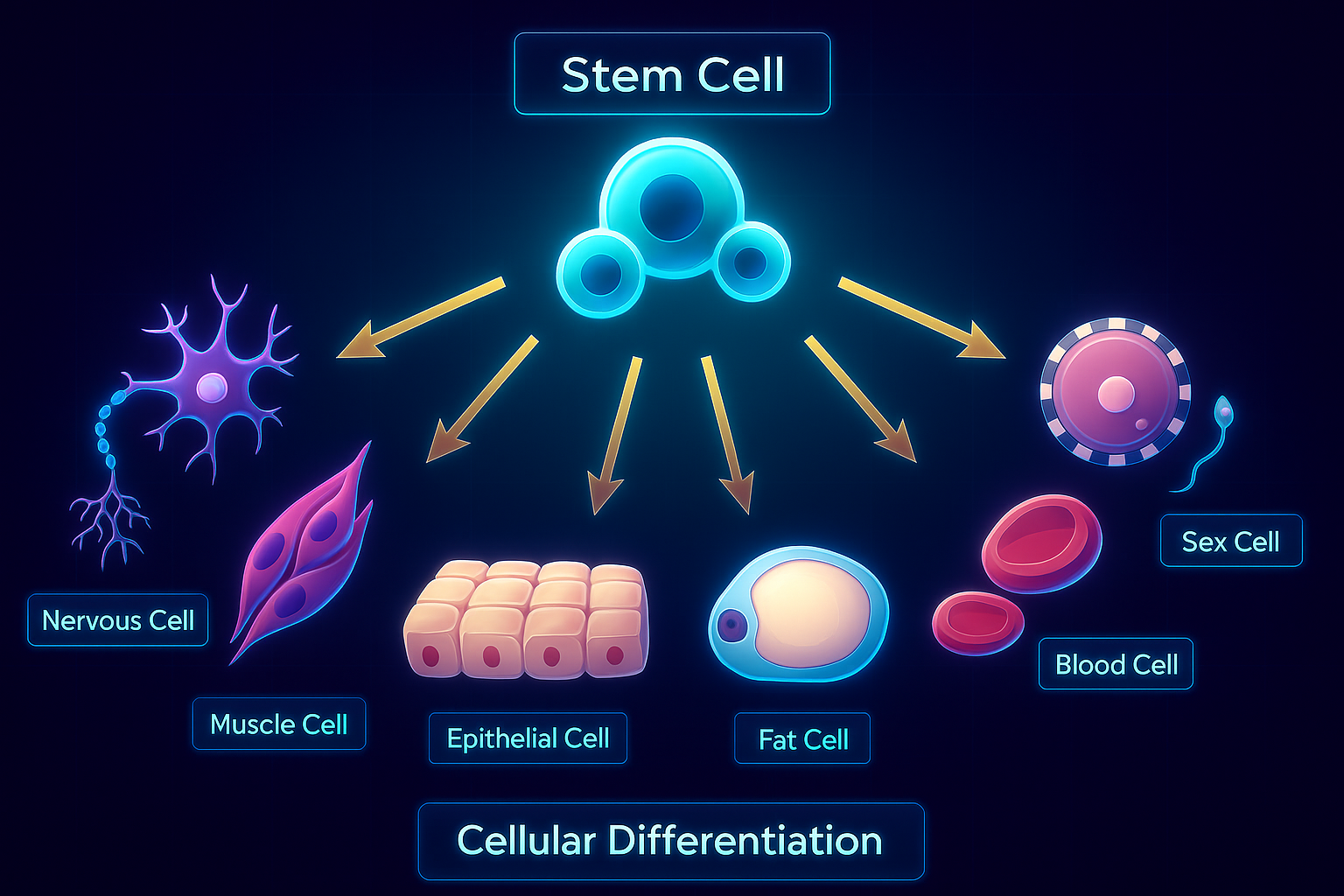
The Epigenetic Symphony: Why Stem Cells Are So Versatile
What gives stem cells their remarkable adaptability is their epigenetic state, the set of chemical markers that tell genes when to turn on or off.
In a mature cell, the genome is like a sheet of music with only one track open: clear, committed, and unchanging.
But in a stem cell? The music is wide open. It’s like a conductor standing before a full orchestra, with the freedom to cue any instrument at any time. This epigenetic openness allows stem cells to quickly activate different genetic programs depending on the body’s needs.
With aging, this flexibility fades. The epigenetic landscape becomes muddled like a symphony with missing notes or overlapping instruments. Important regenerative genes may be silenced, while unhelpful ones get accidentally turned on. This drift contributes to stem cell exhaustion, loss of identity, and ultimately, aging and tissue decline.
Types of Adult Stem Cells: Your Regenerative Toolkit
There are many types of stem cells in the body, but two of the most important are:
Some tissues (like the gut, skin, and blood) rely heavily on high-turnover stem cells that regenerate constantly. Others (like the brain or heart) have fewer active stem cells, which makes them more vulnerable to long-term damage.
When the System Fails: Stem Cell Exhaustion
Over time, due to DNA damage, oxidative stress, inflammation, and toxic exposure, stem cells begin to falter. They may:
This leads to stem cell exhaustion, a critical hallmark of aging that signals the breakdown of your body’s most fundamental repair system.
As stem cells fail, tissues lose their ability to heal, rejuvenate, and respond to stress. The results: thinner skin, weakened immunity, bone loss, muscle wasting, and cognitive decline, the classic signs of aging and regenerative collapse.
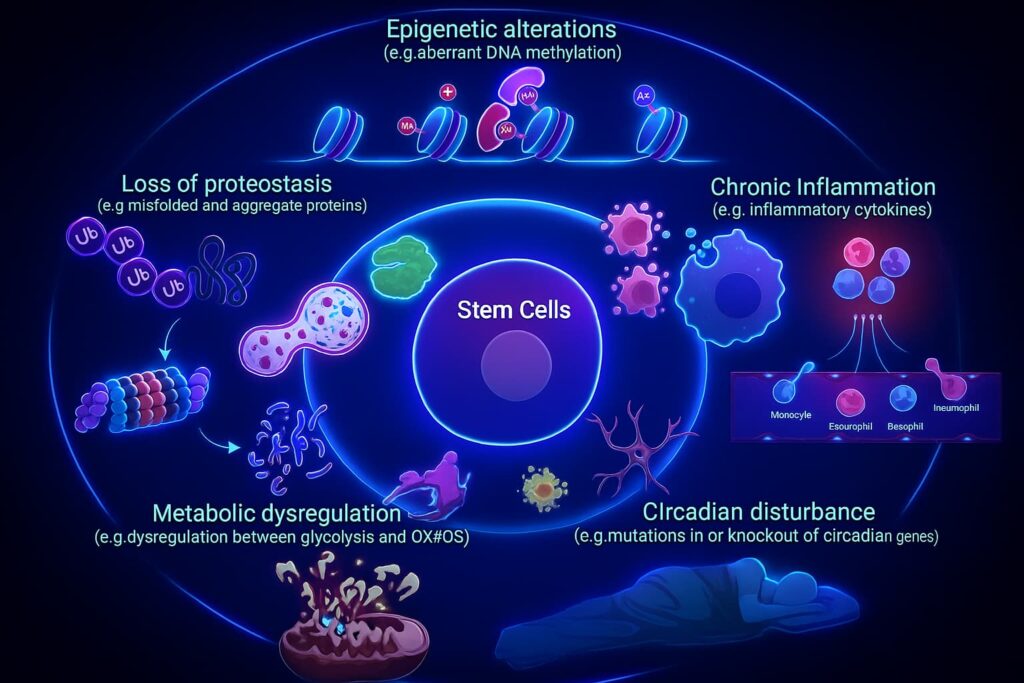
Stem cell exhaustion doesn’t happen overnight, it’s the slow result of both internal wear and tear and relentless external stress. Over time, even the most resilient members of your cellular repair crew start showing signs of fatigue.
What’s driving stem cells toward retirement?
1. DNA Damage: The Blueprint Breaks Down
Every time a stem cell divides, it risks copying errors, small genetic typos that slowly add up. Over the decades, this leads to DNA alterations, weakened repair systems, and telomere shortening like the fraying ends of shoelaces, telomeres shorten with each division, eventually signaling cells to stop dividing altogether. Combined, this DNA damage compromises the stem cell’s ability to function, triggering cell cycle arrest or even cell death.
2. Oxidative Stress: Rust from the Inside
Your mitochondria, the cell’s power generators, also produce reactive oxygen species (ROS) as a byproduct. Normally, antioxidant systems keep ROS in check. But with age, those defenses wear thin.
ROS behave like biochemical sparks, damaging everything from DNA to the mitochondria themselves. Chronic oxidative stress erodes stem cell health, leading to mitochondrial dysfunction, slower energy production, and reduced regenerative capacity like trying to rebuild a house with a broken power drill.
3. Senescence Signaling: Bad Neighbors with a Megaphone
As senescent cells accumulate in aged tissues, they secrete the infamous SASP, a toxic soup of inflammatory cytokines, proteases, and growth factors. These signals don’t just affect surrounding tissues, they also disrupt stem cell signaling. It’s like trying to concentrate in a room full of shouting people: stem cells exposed to SASP lose their focus, slow down their renewal, and may enter premature senescence themselves. This is the cellular version of a toxic neighborhood.
4. Epigenetic Drift: When Instructions Get Blurry
Stem cells rely on epigenetic markers, chemical tags on DNA and histones, to tell them which genes to turn on or off. These markers are what keep stem cells flexible and responsive. But with age, this elegant system begins to drift. Genes meant to stay silent become active, while vital repair genes grow quiet. The result? Stem cells become confused, sluggish, and less capable of responding to stress.
This epigenetic noise is one of the most subtle yet powerful contributors to stem cell exhaustion and aging at the gene regulation level.
5. Niche Degradation: The Stem Cell’s Home Falls Apart
Stem cells don’t float freely, they reside in specialized microenvironments known as niches. These niches provide the biochemical cues, physical support, and immune protection that stem cells need to stay healthy and quiescent until called upon.
But with aging, these niches themselves degrade, due to fibrosis, inflammation, or altered signaling. It’s like living in a once-cozy house that’s falling into disrepair. Even healthy stem cells can’t thrive if their home is crumbling around them.
Together, these forces conspire to weaken stem cell resilience, shrink the regenerative reservoir, and slow down tissue repair across the board. The end result? Stem cell exhaustion, a hallmark of aging that accelerates everything from muscle loss to immune decline and poor wound healing.
Stem cells are your body’s in-house repair crew, specialized, tireless, and on-call for emergencies. But with age, this crew starts to shrink, slow down, or retire altogether.
That’s stem cell exhaustion, one of the key hallmarks of aging. And when it hits, the regenerative engine stalls, leading to a ripple of breakdowns across your body’s tissues and organs.
How this silent decline affects everything from your skin to your brain?
1. Thinner Skin and Delayed Wound Healing
As Skin stem cells become exhausted, your skin loses its regenerative spark. Cuts take longer to close, bruises hang around for weeks, and the skin becomes fragile and paper-thin.
Collagen production slows, elastic fibers degrade, and hydration vanishes, classic signs of aging skin caused by stem cell dysfunction and impaired tissue repair.
Biology bonus: Decreased keratinocyte turnover, reduced fibroblast signaling, and mitochondrial decline in skin stem cells all contribute to cellular aging of the skin.
2. Bone Loss and Joint Degeneration
In the bone marrow, mesenchymal stem cells (MSCs) are responsible for producing bone, cartilage, and connective tissue.
With stem cell exhaustion, they lose their ability to form connective tissues and instead start producing fat. This shift leads to osteoporosis, fragile joints, and a higher risk of fractures and arthritis.
3. Weakened Immune System and Blood Cell Decline
Your hematopoietic stem cells (HSCs) are the source of every immune cell and red blood cell you have.
As they age, HSCs produce fewer lymphocytes and more short-lived, pro-inflammatory myeloid cells, a shift that leads to immune system aging, poor infection control, and slower recovery.
Result: Fewer T cells, impaired B cell memory, and reduced vaccine response, all symptoms of immunosenescence, driven by stem cell depletion.
4. Muscle Wasting and Sarcopenia
Satellite cells, the stem cells responsible for muscle regeneration, become sluggish with age.
Their exhaustion leads to muscle wasting in aging, also known as sarcopenia, reducing strength, balance, and mobility. Small tears in muscle tissue go unrepaired, and muscle fibers shrink over time.
Biological drivers: Oxidative stress, mitochondrial dysfunction, and disrupted Notch signaling pathways hamper satellite cell renewal and muscle repair.
5. Neurodegeneration and Cognitive Decline
Even the brain isn’t spared. Neural stem cells that help generate new neurons and maintain plasticity become fewer and less active with age.
This contributes to age-related cognitive decline, memory loss, and increased risk of neurodegenerative diseases like Alzheimer’s and Parkinson’s.
Mechanism: Chronic inflammation, loss of neurotrophic factors, and DNA damage impair hippocampal neurogenesis and stem cell survival in the brain.
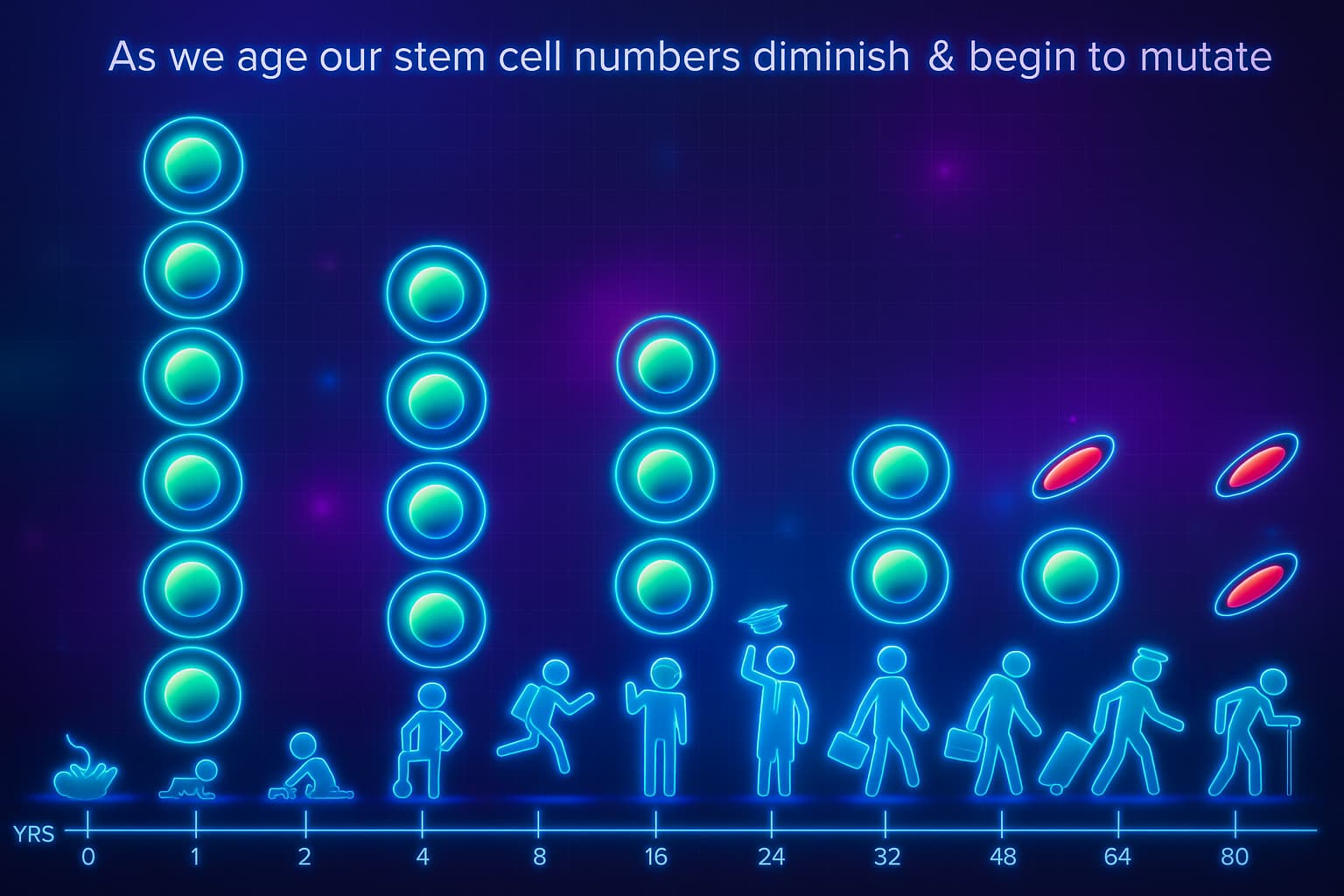
The Domino Effect of Aging and Tissue Repair
When stem cell function declines, so does your body’s ability to heal, renew, and adapt.
Tissues that once turned over effortlessly, skin, muscle, blood, and gut lining, now struggle to keep up. The repair system falters, and biological aging accelerates across the board.
This isn’t just wear and tear, it’s a systemic loss of regenerative potential driven by stem cell aging, mitochondrial dysfunction, and oxidative stress.
Stem cell exhaustion isn’t inevitable and it isn’t irreversible. While aging naturally wears down the body’s regenerative systems, there’s a lot we can do to protect and even rejuvenate our stem cells.
From movement to molecules, certain lifestyle habits and targeted supplements can help preserve stem cell function, delay regenerative decline, and extend healthspan. Think of it as giving your internal repair crew better tools, clearer instructions, and a cleaner work environment.
Let’s explore how daily choices can tip the balance toward longevity and tissue repair and keep your stem cells from burning out before their time.
1. Exercise for Longevity and Stem Cells
Regular physical activity, especially aerobic and resistance training, stimulates the activation and proliferation of tissue-specific stem cells. This helps slow stem cell exhaustion, supports tissue repair, and delays regenerative decline. Exercise is one of the most evidence-backed tools for promoting longevity and stem cell vitality, especially in aging muscles and the aging brain.
2. Intermittent Fasting & Caloric Restriction
These metabolic interventions activate pathways like AMPK and sirtuins, which protect stem cells from oxidative damage and promote autophagy. In models of aging, fasting has been shown to preserve stem cell pools and delay regenerative exhaustion, keeping the body more resilient against age-related decline.
3. Sleep and Tissue Regeneration
Deep, consistent sleep is essential for longevity and tissue repair. During sleep, the body releases hormones that aid in DNA repair and protect against mitochondrial dysfunction, both of which are key to preventing stem cell exhaustion. Poor sleep, on the other hand, accelerates stem cell aging and impairs the regenerative capacity of vital organs.
4. Anti-Inflammatory Diet to Combat Stem Cell Decline
Chronic inflammation is a major driver of stem cell exhaustion and regenerative failure. Diets rich in polyphenols, antioxidants, and healthy fats, like those found in turmeric, green tea, leafy greens, and omega-3-rich fish, create a cellular environment that protects stem cells from aging-related damage. Nutritional strategies are key for supporting stem cells and aging healthfully.
1. Fisetin
Shown to reduce markers of cellular senescence and support stem cell renewal, fisetin helps protect against regenerative decline. It may delay stem cell exhaustion in aging tissues by clearing away the harmful signaling from nearby senescent cells.
2. Resveratrol
This sirtuin-activating compound enhances mitochondrial function and has been shown to extend stem cell lifespan. In models of stem cell aging, resveratrol improves resilience, reduces oxidative stress, and supports tissue repair.
3. Nicotinamide Riboside (NR)
By increasing NAD+ levels, NR supports mitochondrial health, energy metabolism, and DNA repair, all essential for maintaining healthy, functional stem cells over time. It’s a promising tool in delaying stem cell exhaustion and supporting longevity.
4. Melatonin
Often known for regulating circadian rhythm, melatonin also acts as a powerful mitochondrial antioxidant. It shields stem cells from oxidative stress, supports their survival during aging, and may help prevent early onset of regenerative decline.
Not medical advice. Always check with your doctor before using any supplement.
SWhen it comes to reversing regenerative decline and restoring tissue resilience, stem cell-based therapies are at the cutting edge of medical innovation. They hold the potential to not just treat, but truly rebuild the body from within.
Here are some of the most exciting developments:
Autologous Stem Cell Infusions
Using your own adult stem cells (often from bone marrow or adipose tissue), researchers expand these cells in the lab and reintroduce them into damaged tissues. This method may help replenish exhausted cell pools and reignite stalled tissue repair.
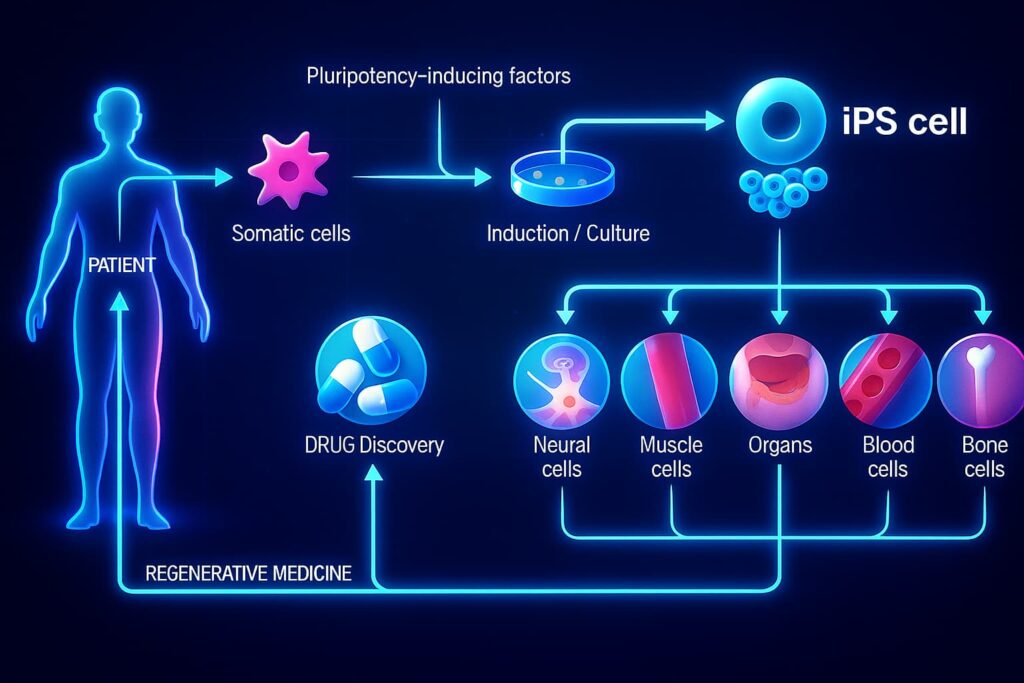
Induced Pluripotent Stem Cells (iPSCs)
iPSCs are adult cells that have been reprogrammed back into a pluripotent, stem-like state using four specific genes, the same Yamanaka factors that earned a Nobel Prize for their discoverer. These reprogrammed cells regain the ability to become virtually any cell type in the body, offering immense potential for regenerative therapies. iPSCs are already used to model diseases, test new treatments, and explore tissue regeneration, with exciting implications for reversing stem cell exhaustion, combating regenerative decline, and extending longevity through personalized cellular repair.
Gene Editing + Stem Cell Transplantation
By combining CRISPR and other tools with stem cells, researchers are beginning to treat inherited blood diseases like sickle cell anemia and beta-thalassemia and this is just the beginning.
These experimental therapies are still in their early stages, mostly limited to clinical trials or highly specialized applications. But the long-term vision is powerful:
Imagine replenishing a failing heart, regenerating nerve cells after injury, or rewinding age-damaged tissues to a more youthful state, not with drugs, but with your own rejuvenated cells.
As we learn more about stem cell exhaustion, stem cells and aging, and the signals that control their fate, the path to meaningful longevity and deep biological repair becomes clearer. The dream?
To one day move from managing aging… to repairing it.
Stem cell exhaustion is a pivotal hallmark of aging, a clear sign that the body’s regenerative engine is running low on fuel. As the internal repair crew weakens, tissues lose their ability to regenerate, leading to slower healing, thinner skin, weaker bones, declining immunity, and even cognitive decline.
This post explores how stem cells and aging are deeply intertwined, revealing the biological causes behind regenerative decline: DNA damage, oxidative stress, chronic inflammation, epigenetic drift, and the breakdown of stem cell niches. Together, these forces drain the stem cell reservoir, accelerating aging and diminishing resilience.
But there’s hope. From intermittent fasting and exercise to anti-inflammatory diets and stem cell-supporting supplements like fisetin, resveratrol, and NAD+ boosters, there are science-backed ways to delay stem cell exhaustion and protect your body’s capacity for tissue repair.
We also highlight breakthroughs in stem cell therapies, including iPSCs reprogrammed with Nobel Prize-winning Yamanaka factors, which may someday reverse tissue aging at the source.
Bottom line? Stem cells are at the heart of longevity and regeneration. Protecting them isn’t just about staying young, it’s about staying capable, resilient, and fully alive.
Now that you’ve unlocked the science behind Stem cell exhaustion, why stop here? The aging process is a mosaic and Stem cell exhaustion is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?