Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“We do not grow old. We grow oxidized, inflamed, and burdened with senescent cells.“
Welcome, explorer of life’s hidden frontiers.
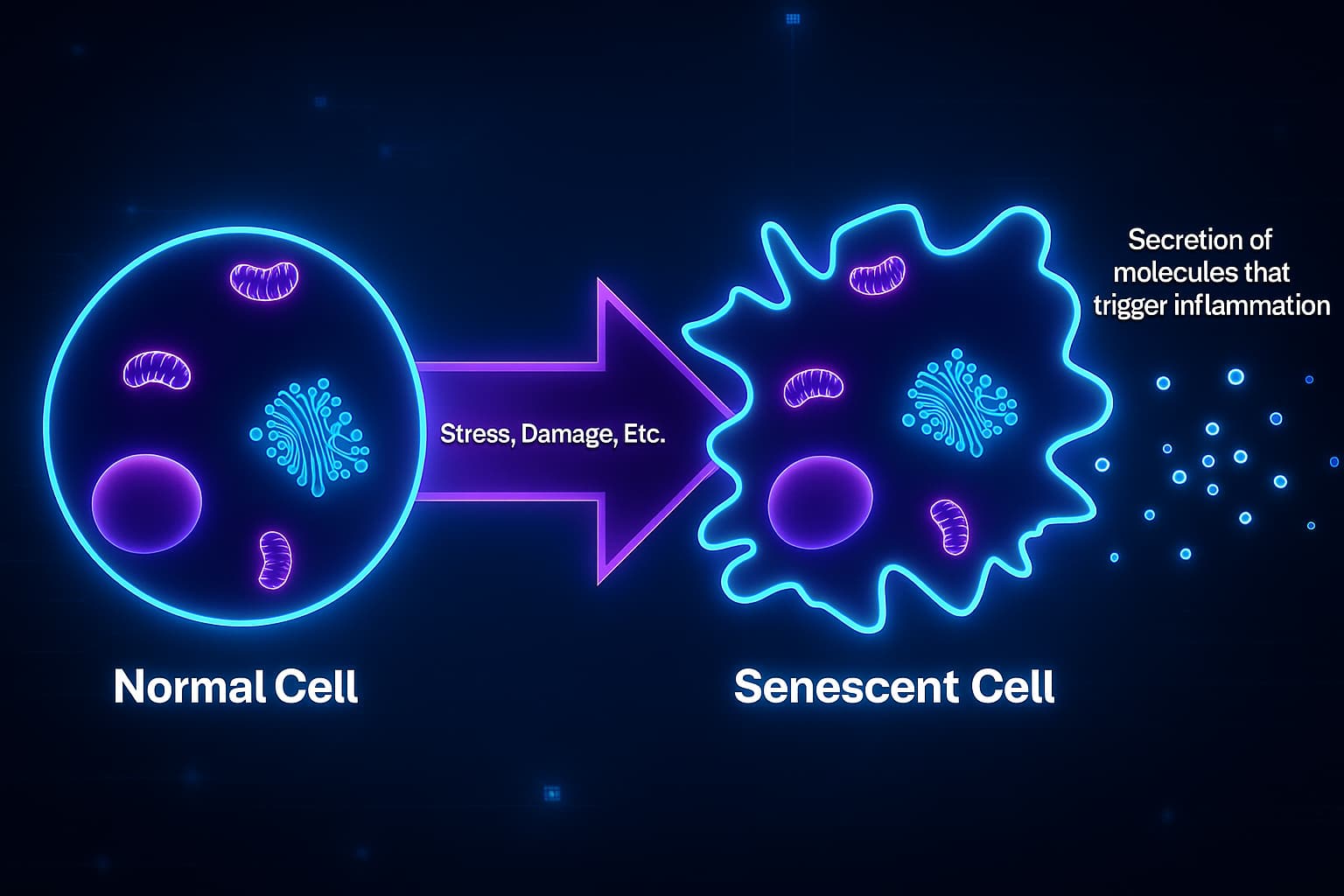
If you have been following this journey through the hallmarks of aging, you know aging is not just about wrinkles or slower reflexes. It is about the silent transformations happening deep inside your cells. Among the most unusual and disruptive of these is cellular senescence, the state where cells stop dividing, stop repairing, yet refuse to die.
The connection between cellular senescence and aging is striking. Senescent cells are not healthy, but they are not entirely dead either. They linger in a dysfunctional limbo, secreting harmful signals that spread inflammation, damage surrounding cells, and accelerate tissue decline. Over time, they accumulate, fueling a cycle that drives many of the symptoms we associate with aging, from immune weakness to fibrosis and frailty.
In this exploration, we will uncover:
To understand cellular senescence and aging is to see how lingering cells can erode vitality from within. Learning how to clear or reprogram them may be one of the keys to healthier, longer life.
Let us uncover the world of zombie cells and explore how removing them could transform the future of longevity.
Cellular senescence is when a cell, often a stem cell or a cell with high replicative potential, hits the brakes and permanently stops dividing. It’s like the cell puts up a “Do Not Disturb” sign and enters a long-term retirement mode.
That might sound fine. After all, a little rest never hurt anyone, right?
But these cells don’t just rest. They refuse to die.
They hang around the tissue like retired workers who still come into the office, hog the coffee machine, and complain loudly about how things used to be done.
Biologically, these senescent cells have exited the cell cycle for good, no more mitosis, no more helping with growth, repair, or regeneration. Worse, they become active disruptors.
Actually… cellular senescence isn’t all bad. In fact, it’s one of your body’s oldest and smartest defenses against cancer.
When a cell faces serious danger, like DNA damage, oxidative stress, or a potentially cancer-causing mutation, it doesn’t roll the dice and keep dividing. Instead, it pulls the plug on itself.
It activates the senescence program, a kind of biological emergency brake, freezing its own ability to replicate.
Imagine a car skidding toward a cliff, hitting the brake might be rough, but it beats going over the edge.
This safety mechanism is critical for preventing tumors, because many cancers begin when damaged cells divide uncontrollably. Senescence says:
“Nope. Not on my watch.”
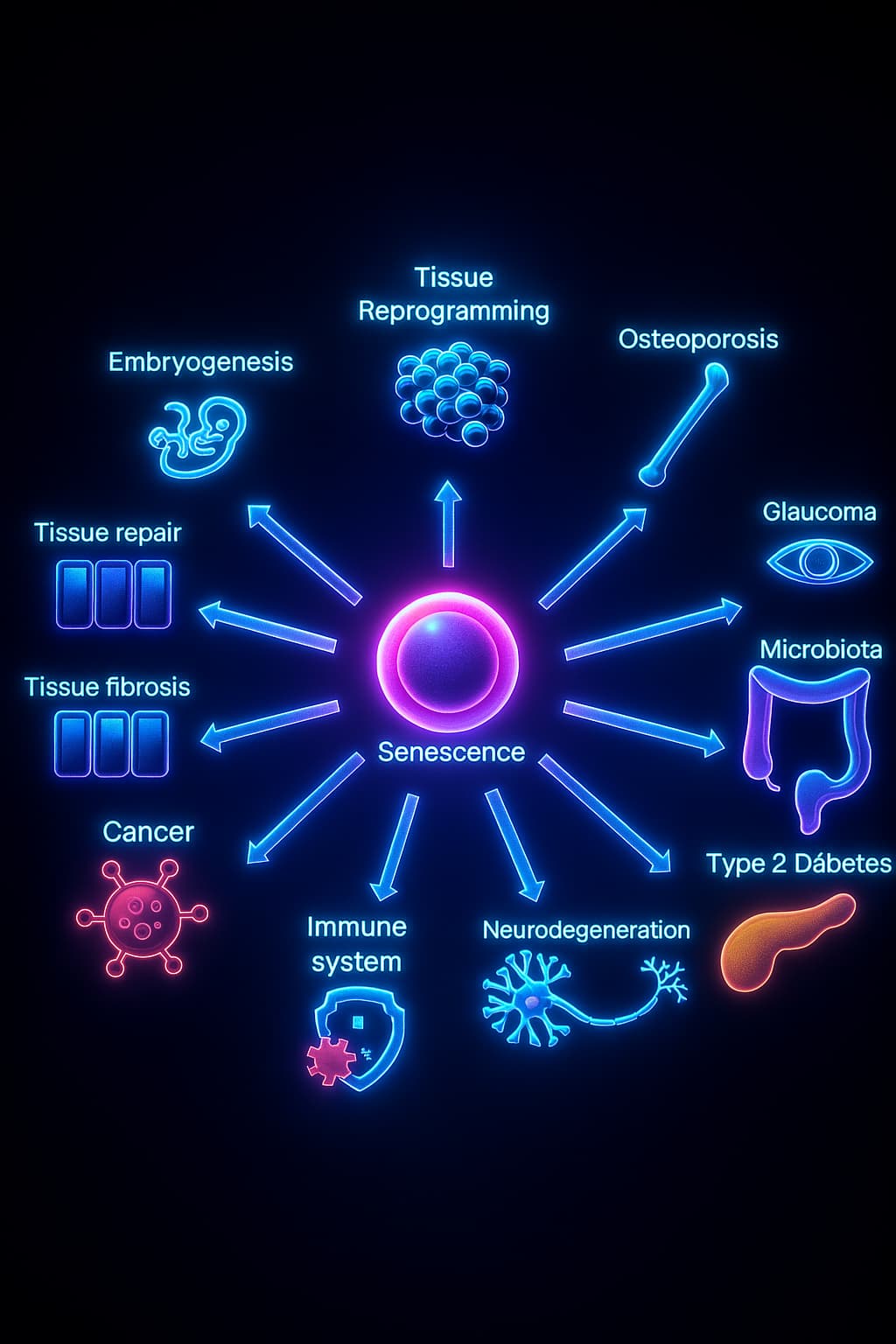
Senescence also plays important roles in:
So yes, senescence is a clever evolutionary trick, not a design flaw.
The problem comes when these cells don’t leave.
What should be a short, controlled pause becomes a long-term breakdown.
The emergency brake stays on… even when the danger has passed. And that’s when senescence flips from protector to saboteur.
As we get older, something strange starts happening inside our tissues: senescent cells begin to pile up, especially in places like your skin, fat, joints, blood vessels, and even your brain.
Why the sudden crowd?
The Immune System Slows Down
When we’re young, the immune system acts like a vigilant janitor, swiftly clearing out damaged or senescent cells. But with age, this cleanup crew weakens. Macrophages and natural killer cells lose efficiency, and over time, more zombie cells slip through the cracks and accumulate.
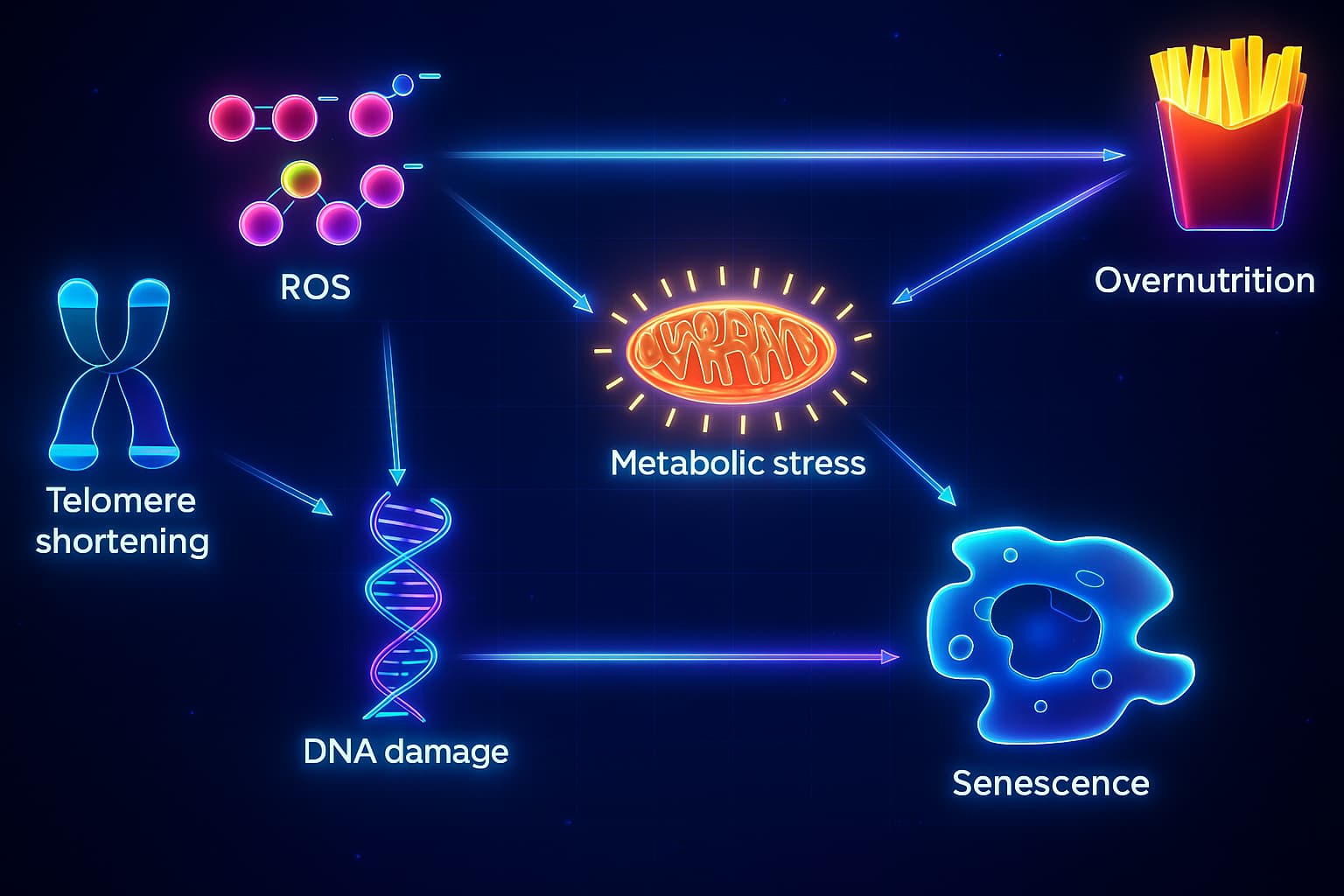
Genomic Instability Increases
Your cells face daily attacks, from UV radiation, pollutants, toxins, and even internal stress. These insults damage DNA, leading to genomic instability, one of the earliest hallmarks of aging. When a cell’s DNA is too compromised to safely divide, it triggers cellular senescence as a protective backup plan.
Telomere Attrition Triggers a Stop
Every time a cell divides, the ends of its chromosomes (called telomeres) get shorter. Eventually, they wear down completely, leaving the genome exposed. This state of telomere attrition is a well-known trigger for senescence, forcing cells to shut down their replication cycle to avoid genomic chaos.
Mitochondrial Dysfunction Fuels Damage
Aging mitochondria, your cells’ energy producers, become leaky and inefficient. They release reactive oxygen species (ROS) that damage nearby structures, including DNA and membranes. This increase in oxidative stress, tied closely to mitochondrial dysfunction, pushes more cells into a senescent state.
Lifestyle Accelerates the Load
Modern habits, poor sleep, inflammatory diets, chronic stress, and inactivity, don’t just wear you down; they accelerate nearly every hallmark of aging. They amplify oxidative stress, disrupt nutrient sensing, and impair immune function, all of which contribute to the buildup of senescent cells.
The result?
A growing crowd of cells that are no longer doing their jobs and worse, they refuse to leave.
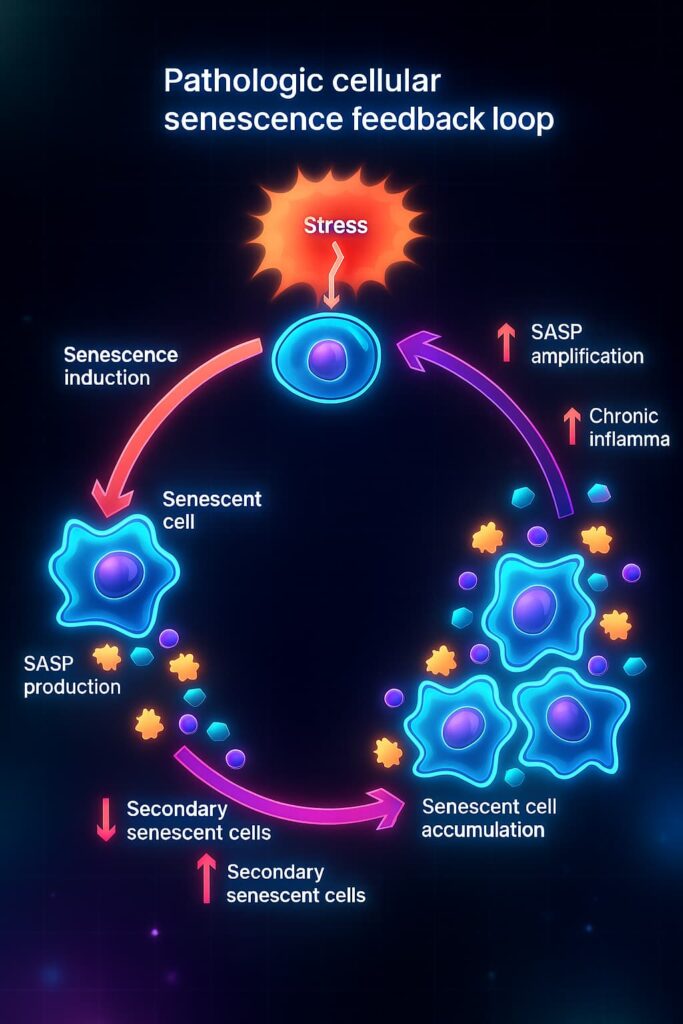
And they’re far from quiet. These cells secrete a toxic stew of inflammatory cytokines, proteases, and misfired signals, collectively known as the senescence-associated secretory phenotype (SASP). The SASP damages nearby tissues, spreads dysfunction, and keeps the inflammation fire burning.
That’s why researchers call them zombie cells:
They’re stuck in limbo, not dividing, not dying, and slowly corrupting the cells around them.
So while cellular senescence begins as a safeguard, its unchecked buildup becomes a major contributor to tissue aging, chronic inflammation, and functional decline.
If senescent cells are like retired workers who won’t leave the office, then imagine they’ve also started blasting angry music and spraying graffiti on the walls.
They’re not just inactive, they’re disruptive.
Biochemically, these “zombie” cells pump out a storm of signaling molecules known as the Senescence-Associated Secretory Phenotype (SASP). It’s not a polite whisper. It’s more like a biochemical megaphone and the whole neighborhood can hear.
So what’s in their toxic mix?
This isn’t just background noise, the SASP is a molecular troublemaker, spreading dysfunction like a bad mood in a crowded room.
Over time, this chemical chaos drives a slow-burning process called inflammaging, chronic, low-grade inflammation that simmers beneath the surface of aging tissues.
How Senescent Cells Cause Us to Age
1. Tissue Degeneration
Inflammatory signals and enzymes released by senescent cells erode tissue structure over time. This contributes to hallmark signs of aging like joint stiffness, fragile blood vessels, and thinning skin, much of which traces back to chronic inflammation and a breakdown in intercellular communication.
2. Collagen Breakdown
The SASP includes proteases that degrade the collagen matrix, the structural scaffold of your skin, joints, and connective tissue. This degradation is tightly linked to loss of proteostasis, weakening the architecture that keeps your body firm, flexible, and resilient.
3. Insulin Resistance
Persistent inflammation from senescent cells interferes with insulin signaling, tipping the body toward insulin resistance, metabolic syndrome, and type 2 diabetes. This metabolic shift is a hallmark of deregulated nutrient sensing, linking senescence to broader metabolic decline.
4. Immune System Decline
SASP factors confuse and exhaust immune cells, diminishing their ability to detect cancer, clear infections, or remove other senescent cells. This contributes to altered intercellular communication and a vicious cycle of immune dysfunction, one that grows more severe with age.
5. Stem Cell Exhaustion
Senescent cells send out signals that suppress the activity of nearby stem cells, the very cells responsible for tissue regeneration. Over time, this suppression leads to stem cell exhaustion, reducing your body’s ability to heal, renew, and adapt.
Altogether, these effects drain energy, disrupt cellular harmony, and push the system further into biological decline, from mitochondrial dysfunction and proteostasis loss to stem cell failure.
This is how zombie cells, once protective, become saboteurs, quietly driving the aging process from within.
Senescent cells may look harmless under a microscope. after all, they’re not dividing, not forming tumors, not actively dying. But don’t be fooled.
These “zombie cells” are biologically active troublemakers. They secrete toxic molecules, disrupt tissue structure, and create an environment where other cells begin to falter. Over time, their presence contributes directly to the development of many age-related diseases, including:
Worse still, these effects aren’t isolated.
Senescent cells create a ripple effect, turning nearby healthy cells dysfunctional and recruiting the immune system into a constant state of low-grade inflammation. This chronic “background noise”, known as inflammaging, slowly wears down your tissues, your immunity, and your ability to repair.
Your daily habits have a powerful say in how many senescent cells build up, and how fast they turn from helpful into harmful. Some choices accelerate the march toward cellular dysfunction. Others slow it down or even help reverse the damage.
These actions support mitochondrial health, strengthen immune surveillance, and enhance cellular resilience.
1. Exercise Regularly
Resistance training and HIIT activate mitochondrial biogenesis, boost autophagy, and recruit immune cells to clear out dysfunctional cells.
2. Embrace Fasting or Caloric Restriction
Intermittent fasting triggers AMPK and sirtuins, which reduce oxidative stress and promote mitophagy, essential for preventing senescent cell buildup.
3. Eat Antioxidant-Rich Foods
Polyphenol-rich plants (green tea, berries, turmeric, leafy greens) reduce ROS, calm inflammation, and suppress the harmful SASP (Senescent Toxic Secreted Mix) signals released by senescent cells.
4. Prioritize Deep Sleep
Good sleep increases melatonin (a powerful mitochondrial antioxidant) and supports immune cells like NK and T cells, your body’s built-in senescent cell assassins.
While lifestyle is the foundation, targeted supplements can give your mitochondria and immune system a gentle push.
1. Fisetin
A natural senolytic found in strawberries and apples. It reduces SASP (Senescent Toxic Secreted Mix), clears senescent cells, and protects tissue, especially in brain and fat tissue in animal studies.
2. Curcumin
The bright compound in turmeric that inhibits NF-κB, lowers ROS, and protects mitochondrial DNA, guarding against premature senescence.
3. Resveratrol
Found in grapes and red wine. Activates sirtuins, boosts mitochondrial performance, and extends healthy cellular lifespan.
4. Melatonin
More than a sleep aid, melatonin stabilizes mitochondrial membranes, lowers oxidative damage, and supports immune cleanup functions.
Not medical advice. Always check with your doctor before using any supplement.
Bottom line?
Small, consistent choices can keep your cellular environment cleaner, younger, and more resilient. Supporting your mitochondria and managing senescent cells doesn’t require perfection, just direction.
Would you like to continue with a detailed section on senolytic therapies and future treatments, or move toward the summary and longevity perspective?
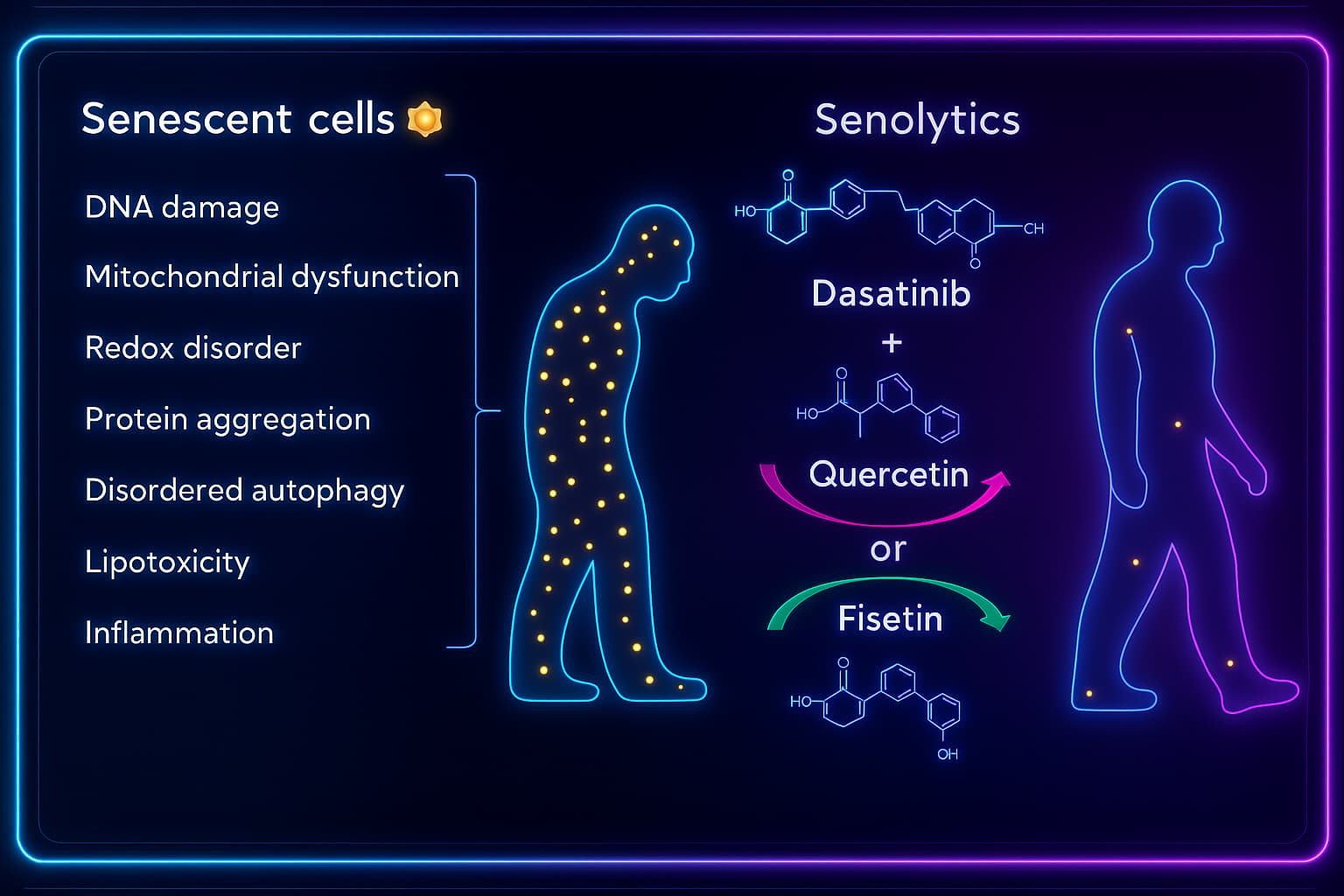
Senolytics are a new class of compounds that specifically target and destroy senescent cells, without Senescent cells aren’t just idle, they’re actively harming the healthy cells around them.
That’s why scientists are developing senolytics, therapies designed to selectively destroy senescent cells while sparing healthy ones. Think of them as precision cleanup crews that target the bad apples before they spoil the whole bunch.
Some of the most promising senolytic approaches:
Dasatinib + Quercetin
Navitoclax
Originally developed as a cancer drug, navitoclax blocks BCL-2 family proteins, molecules that senescent cells use to avoid death.
Senomorphics (gentler approach)
Rather than killing senescent cells, these compounds suppress the SASP, the inflammatory and tissue-damaging signals senescent cells release.
A New Frontier in Longevity
Senolytic therapies are still in the early stages, but clinical trials are underway, and the results so far are exciting.
In the near future, clearing senescent cells may become as routine as taking a supplement or getting a vaccine.
These interventions have the potential to extend healthspan, delay age-related diseases, and improve quality of life in older adults.
Cellular senescence is like a retirement plan that goes sideways.
In theory, it starts with good intentions, damaged cells step out of the cell cycle to avoid turning cancerous. But instead of quietly bowing out, many of these cells refuse to die. They stick around as zombie cells: inactive, dysfunctional, and dangerously loud.
These lingering cells send out inflammatory distress signals that confuse nearby cells, disrupt tissue function, and fuel chronic conditions, from joint degeneration and cardiovascular disease to neurodegeneration and immune decline. This is how the once-helpful process of cellular senescence and aging becomes a key driver of biological decline.
But it turns out we can intervene.
From cutting-edge senolytic therapies designed to selectively remove senescent cells, to everyday lifestyle strategies like intermittent fasting, exercise, sleep optimization, and antioxidant-rich diets, we’re learning how to reduce the burden of zombie cells and restore cellular harmony.
By protecting mitochondrial health, dialing down inflammation, and supporting stem cell renewal, we can slow the aging process at its roots.
So if longevity is the mission, clearing out the cellular dead weight is part of the plan.
Let’s keep the cellular office clean, efficient, and thriving and make sure those retired cells don’t overstay their welcome at the molecular coffee machine.
Now that you’ve unlocked the science behind Mitochondrial dysfunction , why stop here? The aging process is a mosaic and Mitochondrial dysfunction is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?