Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“You’re only as young as your mitochondria.“
Welcome, traveler into the heart of the cell.
If you have been following this journey through the hallmarks of aging, you know aging is not only about gray hairs or aching joints. It is about the gradual weakening of the deep systems that keep us alive. At the center of this story is mitochondrial dysfunction, the slow breakdown of your cells’ energy generators.
The connection between mitochondrial dysfunction and aging is profound. Mitochondria do more than produce energy. They regulate cell survival, signal repair, and help maintain metabolic balance. When they begin to falter, oxidative stress rises, inflammation spreads, nutrient sensing loses balance, and autophagy slows. Damaged mitochondria accumulate, setting off a chain reaction that erodes cellular resilience and accelerates biological aging.
In this exploration, we will uncover:
To understand mitochondrial dysfunction and aging is to see how the energy of life itself can fade—and how, by preserving these powerplants, we may extend not only our years but the vitality within them.
Let us step inside the cell’s powerhouse and explore how to keep its engines running strong.
Your mitochondria are the microscopic powerplants inside nearly every cell in your body. But calling them powerplants doesn’t quite do them justice.
Yes, they convert the food you eat into ATP (adenosine triphosphate), the molecular fuel that powers everything from heartbeat to brainwaves. But mitochondria are much more than tiny energy generators.
The mitochondria roles in you cells:
Think of mitochondria as the city hall, power station, waste management center, and public safety department of your cells, all rolled into one.
Unexpected twist
mitochondria weren’t always part of us. Scientists believe they started as free-living bacteria billions of years ago, independent organisms that entered a symbiotic relationship with early eukaryotic cells. Over time, this partnership became so essential that the two fused into one. The mitochondria gave us energy and in return, got a place to live. That ancient merger still echoes in your cells today: mitochondria even carry their own tiny strand of DNA, a relic of their bacterial ancestry.
So what happens when these ancient allies start to falter?
Mitochondrial dysfunction doesn’t just lead to fatigue, it disrupts every system they support. From hormone imbalances to impaired immunity, calcium misregulation, oxidative stress, and cell death, the breakdown of mitochondrial health is a multi-system collapse and a key driver of aging and disease.
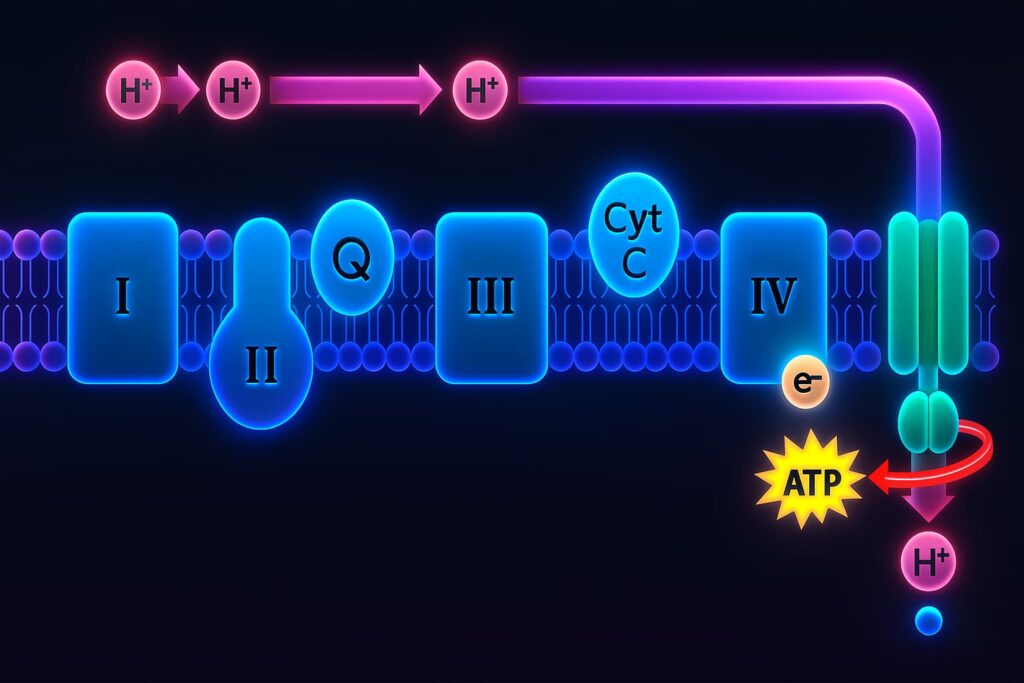
At the heart of every mitochondrion is a process called oxidative phosphorylation, the sophisticated system your cells use to turn food into energy.
But to make sense of it, forget chemistry textbooks for a moment and picture a hydropower dam.
1. The Fuel: Electrons from Food
When you eat, your body breaks down carbohydrates, fats, and proteins into smaller pieces. These are fed into a cycle inside the mitochondria (the Krebs cycle) where they release electrons, tiny packets of energy.
Think of these electrons like water diverted into a steep channel, ready to generate power.
2. The Turbine System: The Electron Transport Chain
Those high-energy electrons are passed through a series of protein complexes embedded in the inner mitochondrial membrane, this is the electron transport chain.
Each step in the chain pumps protons (H⁺ ions) across the membrane, creating a proton gradient a reservoir of stored potential energy, like water building up behind a dam.
3. The Power Surge: ATP Synthase
Once enough protons have piled up, they rush back down through a special turbine-like protein called ATP synthase.
As they flow, they spin the rotor, literally and that mechanical motion powers the production of ATP (adenosine triphosphate), the energy currency of life.
It’s like water spinning a hydroelectric turbine to light up an entire city, only this city is a living cell.
4. The Byproduct: A Little Bit of Chaos
The process isn’t perfect. Occasionally, an electron leaks out and reacts with oxygen, forming reactive oxygen species (ROS), your cellular sparks.
In moderation, ROS are useful messengers. But when too many escape, they start scorching the very machinery that makes energy possible.
In short:
Your mitochondria are molecular dams, turning the flow of electrons into a spinning turbine that powers every heartbeat, thought, and breath.
And the better those turbines run, the more energy your cells have to grow, repair, and stay young.
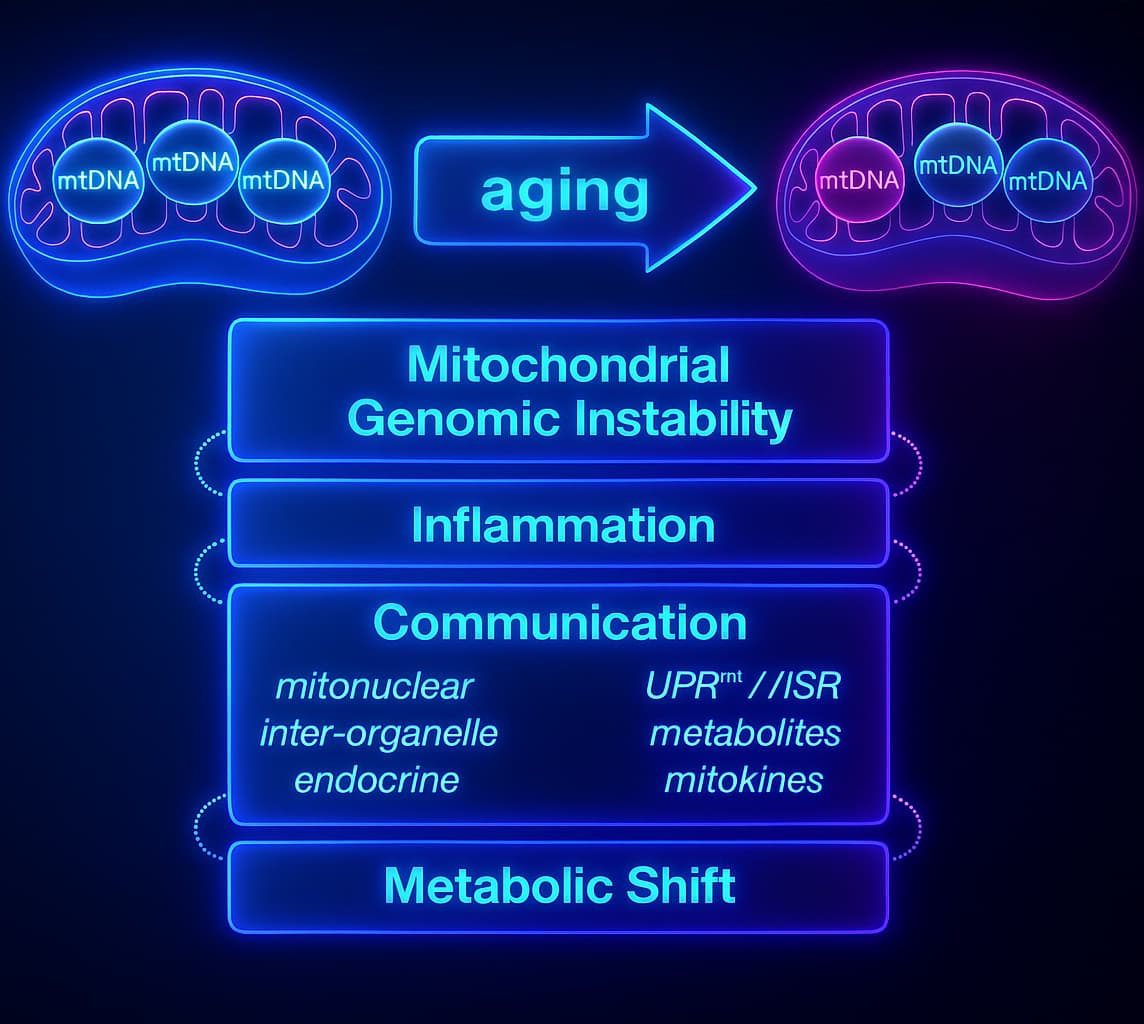
As mitochondria age, they don’t just slow down, they start misfiring, leaking, and dragging the whole system down. But why does this happen? What causes these powerhouses to go rogue?
Let’s break it down:
1. Energy Production Drops
Mitochondria make ATP, your cell’s fuel, through oxidative phosphorylation.
But with age, DNA damage, especially in mitochondrial DNA (mtDNA), messes with the blueprints.
Meanwhile, epigenetic drift and faulty protein assembly mean the electron transport chain runs like a busted power grid. Fewer sparks, less energy.
2. ROS Production Spikes
Normally, mitochondria handle their sparks (ROS) with care. But aging throws a wrench in the system.
Oxidative stress, broken protein parts, and worn-out Complex I and III let electrons leak.
More leaks = more ROS = more chaos. Antioxidant defenses drop, and the sparks start flying.
3. Membranes Get Leaky
Mitochondrial membranes are supposed to be tight, keeping calcium, charge, and death signals in check.
But ROS chew through membrane lipids. Damaged proteins pile up (loss of proteostasis), and the gates start to leak.
It’s like trying to run a spaceship with holes in the hull.
4. Mitochondrial DNA Takes Hits
MtDNA lives dangerously close to the ROS source and it has weak repair tools.
No histones, no armor. Just exposure.
With time, oxidative damage leads to mutations that disrupt key mitochondrial genes and that means more dysfunction, more ROS, more damage.
5. Broken Mitochondria Pile Up
Your cells usually take out the trash through mitophagy, a form of autophagy.
But that system slows with age. Signals fade, recycling breaks down, and damaged mitochondria hang around like broken engines in a junkyard.
This isn’t just clutter it’s toxic, energy-draining clutter.
Mitochondria and Aging: A Downward Spiral
Mitochondrial dysfunction isn’t just a symptom, it’s a central cause of cellular aging, triggering oxidative stress, impaired autophagy, DNA damage, senescence, and more.
When your powerplants break down, the whole city dims.
When mitochondria start to fail, it’s not just an energy issue, it’s a full-blown cellular crisis. Like a control tower losing power, the ripple effects spread far and wide, disrupting metabolism, cell communication, and tissue maintenance. This kind of mitochondrial dysfunction sets off a domino chain of interconnected hallmarks of aging.
1. Autophagy Slows
Healthy mitochondria help kickstart autophagy the cellular cleanup crew that clears out broken proteins and organelles.
But aging mitochondria lose the ability to send those signals. Cleanup stalls, waste builds up, and proteostasis breaks down.
Think of it like a city without garbage trucks, things get toxic fast.
2. Inflammation Spikes
Damaged mitochondria leak mtDNA and stress molecules that trigger the immune system.
This fuels chronic inflammation (aka inflammaging) and throws off intercellular communication — making healthy signals harder to hear.
It’s like a smoke alarm stuck on high, draining your system and causing cellular stress.
3. Insulin Sensitivity Falls
Mitochondria help process glucose and fat, and they play a key role in nutrient sensing.
When they falter, your cells can’t respond properly to insulin, leading to insulin resistance, metabolic dysfunction, and accelerated cellular aging.
4. Stem Cells Burn Out
Stem cells need energy to divide, renew, and repair damaged tissue.
But with rising oxidative stress and falling mitochondrial performance, their output slows, driving stem cell exhaustion and reduced tissue regeneration.
It’s like trying to rebuild a city with no power tools.
5. Cell Death Increases
Mitochondria control the life-or-death decisions inside your cells.
When their membranes become unstable, they can trigger apoptosis or even necrosis.
The result? Accelerated tissue decline, organ dysfunction, and a faster path to biological aging.
The Big Picture: Mitochondria at the Center of Aging
This is why mitochondrial dysfunction and aging are so tightly linked.
From metabolic decline and inflammaging to autophagy failure and stem cell exhaustion, it all traces back to these tiny, overworked powerplants.
Keep them healthy, and you protect your system from the inside out.
They’re not static structures, they adapt, rebuild, and multiply when given the right signals. Think of them like a team of workers who’ve been slacking off, not because they’re lazy, but because they haven’t been challenged in the right way.
The trick isn’t magic. It’s biology. And it starts with how you live, move, eat, and rest.
You don’t need a PhD in biochemistry, just the right cues. These daily choices send mitochondria the m
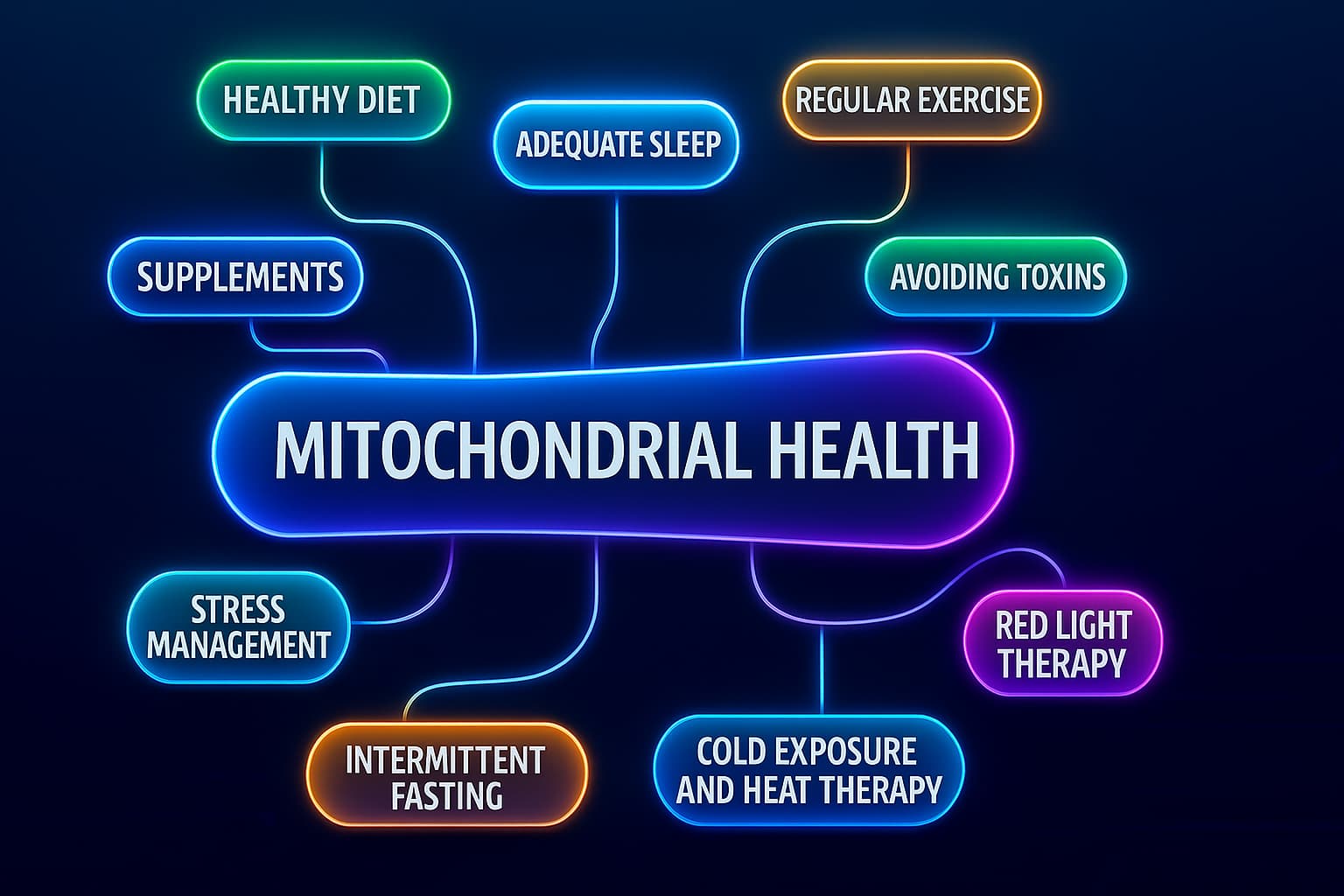
1. Move with Intensity
Exercise, especially HIIT (high-intensity interval training) and resistance training, is one of the strongest triggers for mitochondrial biogenesis (the creation of new mitochondria).
It’s like telling your cells, “We need more power!” and mitochondria respond by building new, more efficient energy factories.
2. Embrace Intermittent Fasting
Fasting activates mitophagy, the cellular recycling process that clears out old, damaged mitochondria to make room for new ones.
You’re not starving, you’re spring cleaning your cells. This process also reduces ROS and improves metabolic flexibility, helping your cells switch between burning carbs and fat for fuel.
3. Prioritize Deep, Consistent Sleep
Your mitochondria are tuned to your circadian rhythm, their repair and maintenance happens largely while you sleep.
Poor sleep means skipped repairs. But quality rest gives mitochondria the chance to reset, restore, and recharge for the day ahead.
4. Try Hormetic Stress (In Small Doses)
Short, controlled stressors like cold exposure, saunas, fasting, or intense exercise trigger adaptive responses that make mitochondria tougher and more efficient.
Think of it as weight training for your mitochondria, a little challenge builds long-term strength and resilience.
1. Magnesium
Helps generate ATP, the cell’s main energy molecule, and stabilizes mitochondrial membranes.
Without enough magnesium, your mitochondria struggle to complete energy production efficiently.
2. B Vitamins (especially B1, B2, B3, B5)
Act as coenzymes in the mitochondrial energy cycle essential for converting food into fuel.
They keep your mitochondrial engines running smoothly and prevent energy bottlenecks.
3. Lipoic Acid
A powerful antioxidant that neutralizes reactive oxygen species (ROS) and recycles other antioxidants like vitamins C and E.
It also supports enzymes in the Krebs cycle, enhancing energy output.
4. Carnitine
Transports fatty acids into mitochondria, where they’re burned for fuel.
Carnitine helps your cells access fat as an energy source, especially during fasting or exercise.
5. Polyphenols
Found in green tea, blueberries, turmeric, and dark chocolate, polyphenols help reduce oxidative stress and activate protective mitochondrial genes.
They act as mild stressors that train mitochondria to adapt and grow stronger, a process known as hormesis.
6. Coenzyme Q10 (CoQ10)
A key part of the electron transport chain, CoQ10 shuttles electrons between protein complexes, enabling ATP production.
It also protects mitochondria from ROS and declines with age, making supplementation especially useful after 40.
7. NAD⁺ Precursors (NR and NMN)
Support levels of NAD⁺, a molecule that delivers electrons to the electron transport chain and activates sirtuins, proteins involved in DNA repair and longevity.
Boosting NAD⁺ helps restore cellular energy, metabolic health, and mitochondrial resilience.
Not medical advice. Always check with your doctor before using any supplement.
Small Changes, Big Energy
You don’t have to do it all at once.
Start with movement. Improve your sleep. Add color to your plate. Maybe introduce a supplement or two.
The message is simple: your mitochondria are listening and ready to respond.
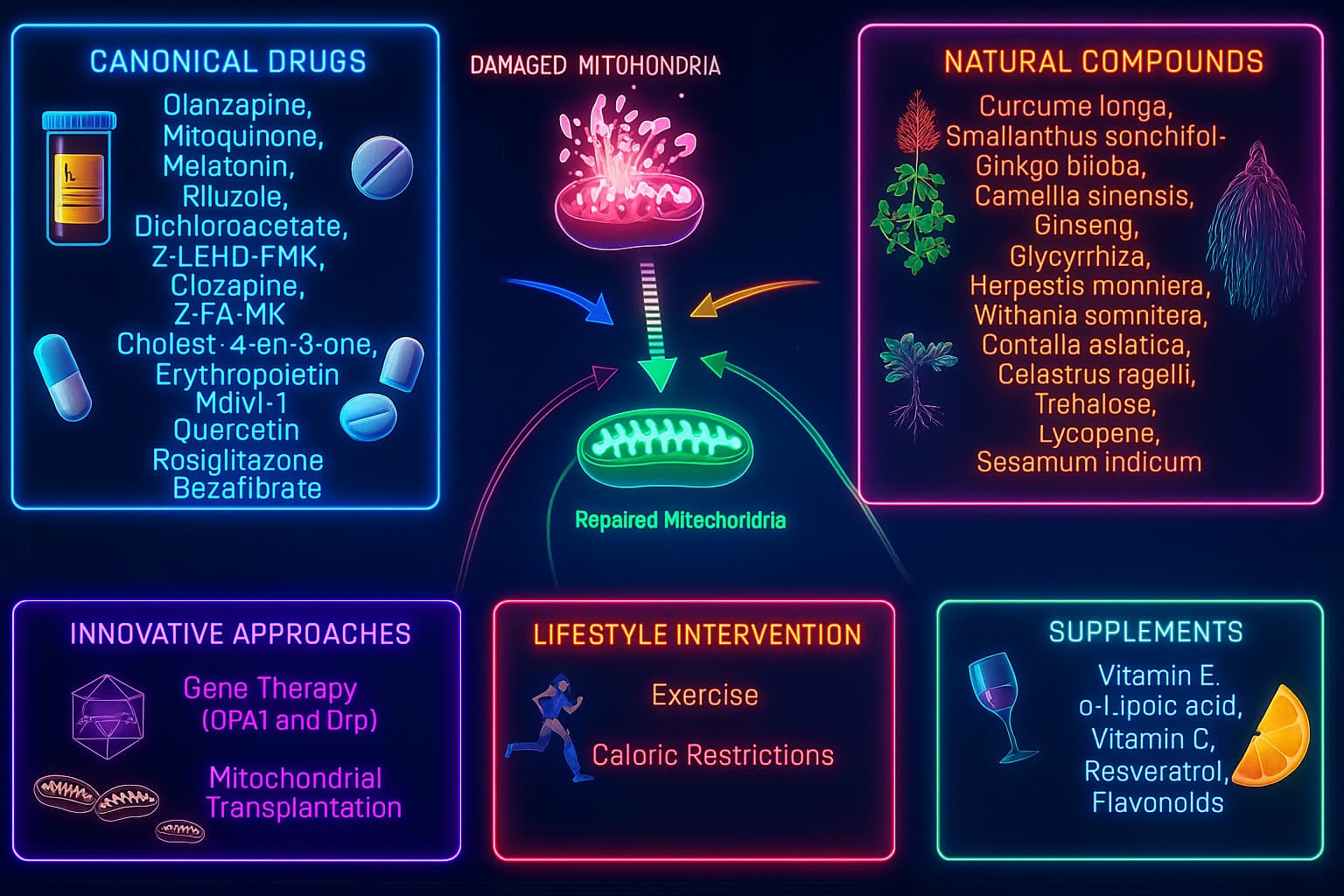
Beyond lifestyle and nutrition, science is entering a new era, one where we can target mitochondria directly with precision tools. These therapies go beyond general wellness and aim to restore mitochondrial health at the molecular level, potentially reversing key aspects of cellular aging.
Here are some of the most exciting frontiers:
1. Mitochondrial Biogenesis Enhancers
Compounds like PQQ (pyrroloquinoline quinone) and exercise-mimicking peptides are being studied for their ability to spark the creation of new mitochondria, essentially increasing your cell’s energy capacity.
Think of it as upgrading your power grid with fresh, high-efficiency generators.
2. Mitochondria-Targeted Antioxidants
Unlike general antioxidants, compounds like MitoQ, SkQ1, and SS-31 are designed to go straight to the mitochondria, where most oxidative stress originates.
They act like fire extinguishers placed right inside the engine room, putting out sparks before they can cause damage.
3. Gene Therapy & Mitochondrial Editing
Early-stage technologies are exploring ways to repair or replace mutated mitochondrial DNA, one of the root causes of mitochondrial dysfunction in aging and inherited disease.
CRISPR-based tools and mitochondrial gene transfer are still experimental, but they hold promise for future therapies that correct dysfunction at the source.
4. Mitophagy Activators
Drugs and compounds that stimulate mitophagy like urolithin A, help your cells identify and recycle defective mitochondria.
It’s like bringing back the cleanup crew to clear out broken machines and keep your energy systems efficient and safe.
The Future Is Mitochondrial
Many of these innovations are in clinical trials or biotech pipelines, with early results showing improved energy metabolism, reduced inflammation, and better physical performance, even in older individuals.
They won’t replace healthy habits but they could supercharge your body’s ability to recover, repair, and thrive.
As the science matures, supporting your mitochondria won’t just mean eating better or exercising more.
It could mean targeted interventions that restore cellular vitality from the inside out.
Mitochondria aren’t just tiny energy factories, they’re at the heart of how and why we age. When mitochondrial function declines, it’s not just about feeling tired, it’s a cascade.
Mitochondrial dysfunction leads to cellular energy loss, chronic inflammation, rising oxidative stress, and impaired repair systems. This breakdown drives nearly every major hallmark of aging, from stem cell exhaustion and impaired autophagy to genomic instability and deregulated metabolism.
It’s no wonder scientists now see mitochondrial health as a cornerstone of healthy aging and longevity.
The good news?
Even in aging cells, mitochondria can recover, regenerate, and rebuild, if we give them the right environment.
How to Support Mitochondrial Health for Longevity:
We live in a world that drains mitochondrial function, processed food, poor sleep, toxins, and sedentary routines all play a role. But the science of mitochondria and aging shows that it’s possible to reverse the trend.
Because the truth is:
The path to a longer, healthier life starts in your cells, and mitochondria are the gatekeepers of that journey.
Now that you’ve unlocked the science behind Mitochondrial dysfunction , why stop here? The aging process is a mosaic and Mitochondrial dysfunction is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?