Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.
“Longevity isn’t about never growing old, it’s about helping your cells remember when to grow, and when to heal.“
Welcome, navigator of the body’s inner compass.
If you have been journeying with us through the hallmarks of aging, you know that the passage of time is not only measured in years. It is etched into the gradual changes of deep cellular systems, the invisible forces that guide how we age from within. One of the most fascinating, yet often underestimated, is deregulated nutrient sensing.
This is the body’s internal navigation system, a network of sensors that tells your cells when to grow, when to repair, and when to conserve. In youth, this balance is precise. With age, the compass drifts. Cells get stuck in constant “grow mode,” consuming energy when they should be restoring. The connection between nutrient sensing and aging lies in this imbalance. When nutrient sensing fails, metabolism falters, inflammation rises, insulin resistance develops, and mitochondria begin to weaken.
The effects ripple through other hallmarks of aging, amplifying autophagy decline, stem cell exhaustion, and chronic inflammation. It becomes a feedback loop that accelerates the loss of vitality.
In this exploration, we will uncover:
To understand nutrient sensing and aging is to see how life constantly chooses between building and preserving. Learning to guide that choice may be one of the most powerful tools for extending health and vitality.
Let us open this control center and explore how tuning it might reshape the course of aging.
Your cells don’t eat pizza or count carbs but they do constantly monitor nutrient levels. This inner intelligence is called nutrient sensing: your body’s built-in radar for detecting fuel, energy balance, and environmental stressors.
It’s how your cells decide whether to grow or repair, store or conserve, build new tissue or recycle damaged parts. This elegant system ensures your body runs efficiently like a conductor orchestrating a symphony of cellular processes.
But with age, this harmony starts to fade. The sensors misfire. The rhythm falters. And this subtle loss of coordination begins to echo across your entire biology.
The Metabolic Control Panel: How Your Cells Make Decisions
At its core, nutrient sensing is a communication network, one that tracks key cellular signals like glucose, amino acids, ATP (energy), and insulin. These signals feed into master regulators that flip molecular switches:
These decisions are guided by a trio of key signaling proteins, the primary switches on your cellular dashboard (don’t worry about the names, they’re just scientific labels):
In youth, this dashboard is finely tuned. Cells flip seamlessly between growth and repair, storage and usage, activity and rest.
At the heart of nutrient sensing and aging lie three master regulators. powerful molecular switches that control whether your body should grow, repair, or conserve energy. In youth, these pathways operate in harmony, shifting effortlessly between building and cleanup depending on your energy needs.
But with time, this delicate balance begins to break down.
As the primary hallmarks of aging, including mitochondrial dysfunction, genomic instability, and chronic inflammation, accumulate, the messages flowing through your cellular signaling networks become distorted. These disruptions lead to deregulated nutrient sensing, where once-adaptive systems start sending the wrong instructions.
Let’s break down the key players and how they lose control as we age.
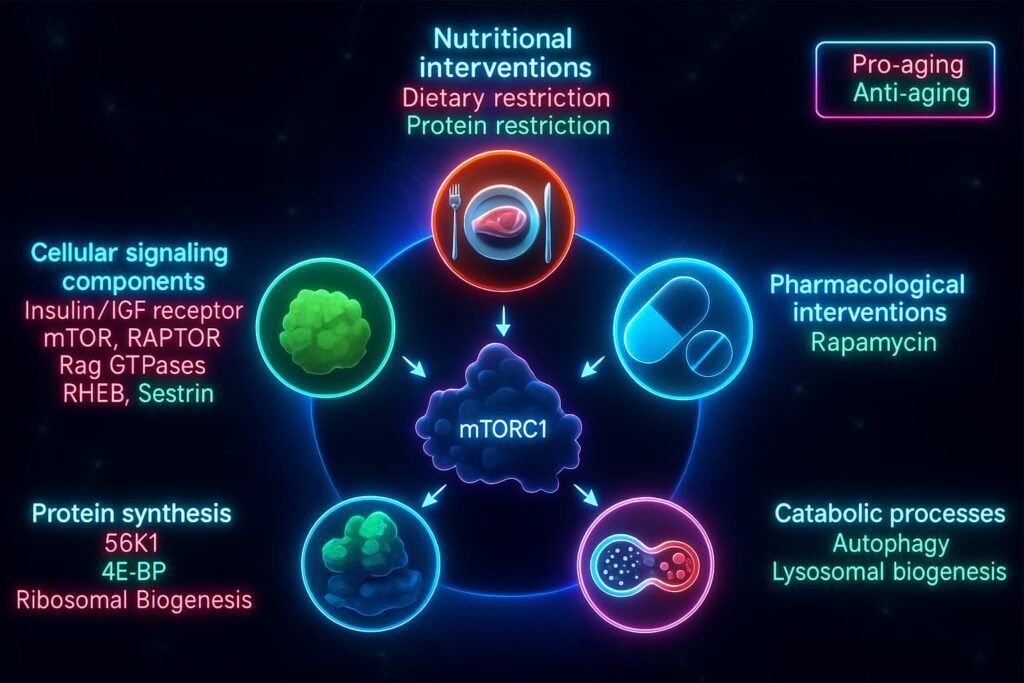
mTOR: The Growth Accelerator That Won’t Let Up
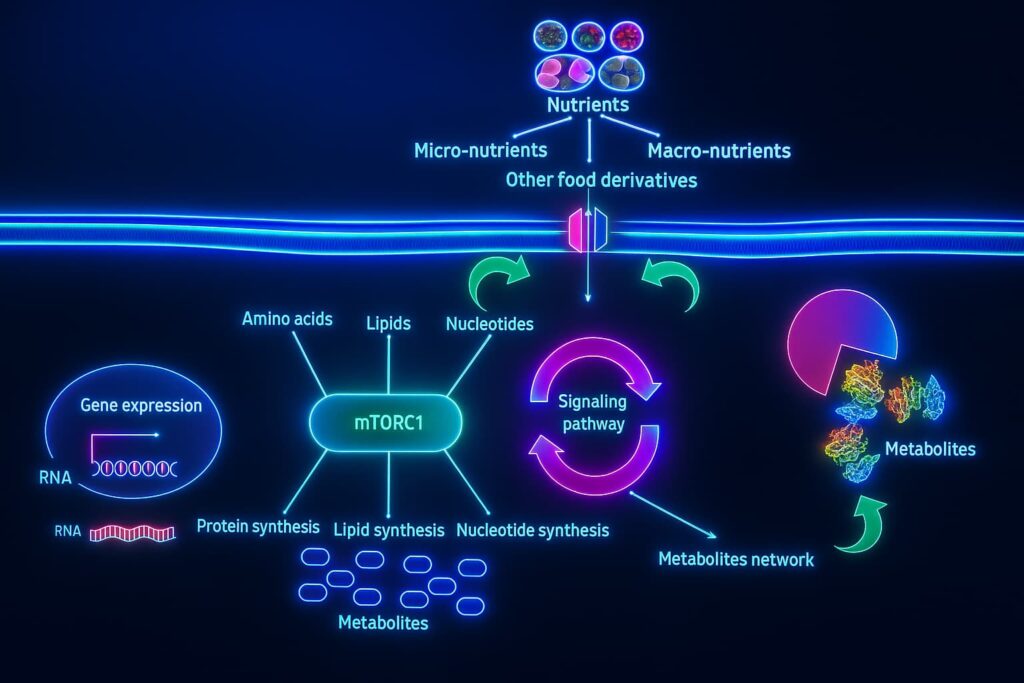
mTOR (mechanistic Target of Rapamycin) acts like your cellular gas pedal. When nutrients like amino acids and glucose are plentiful, mTOR promotes cell growth, protein synthesis, and division, all essential during youth for development and tissue repair.
But with aging, mTOR becomes chronically overactive a key feature of deregulated nutrient sensing.
Why?
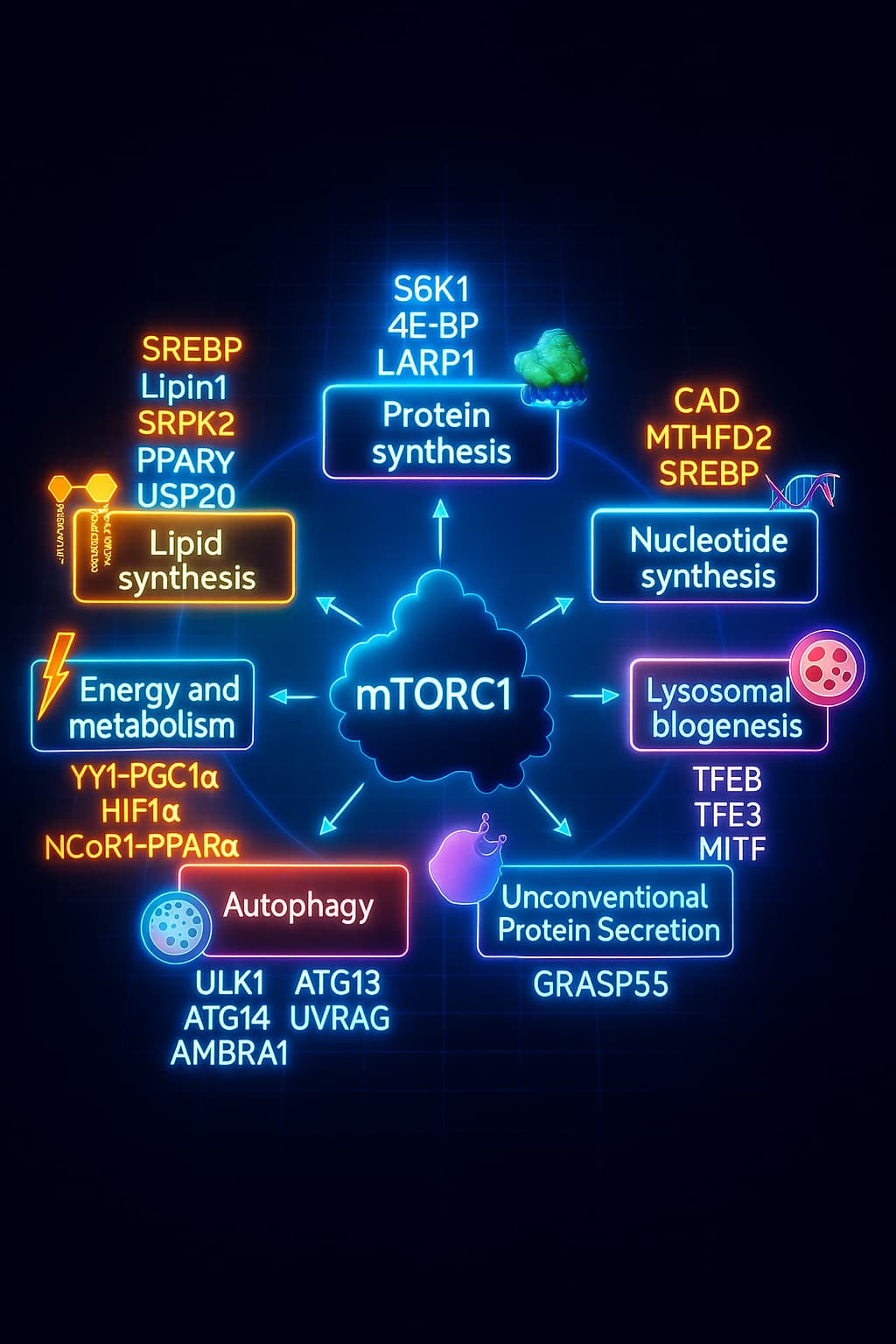
The result? Autophagy is suppressed, inflammation rises, and damaged cells keep growing, all of which accelerate aging and increase cancer risk.
This chronic mTOR activation is a major contributor to the breakdown in autophagy and aging resilience.
AMPK: The Repair Mode That Fades with Age
AMPK (AMP-activated protein kinase) is your cellular emergency brake, a guardian of metabolic flexibility and longevity. It switches on during energy stress, like fasting or intense exercise, signaling your body to conserve resources and initiate repair.
AMPK activates autophagy, enhances mitochondrial biogenesis, and restores energy balance.
But with age, AMPK responsiveness fades.
Why?
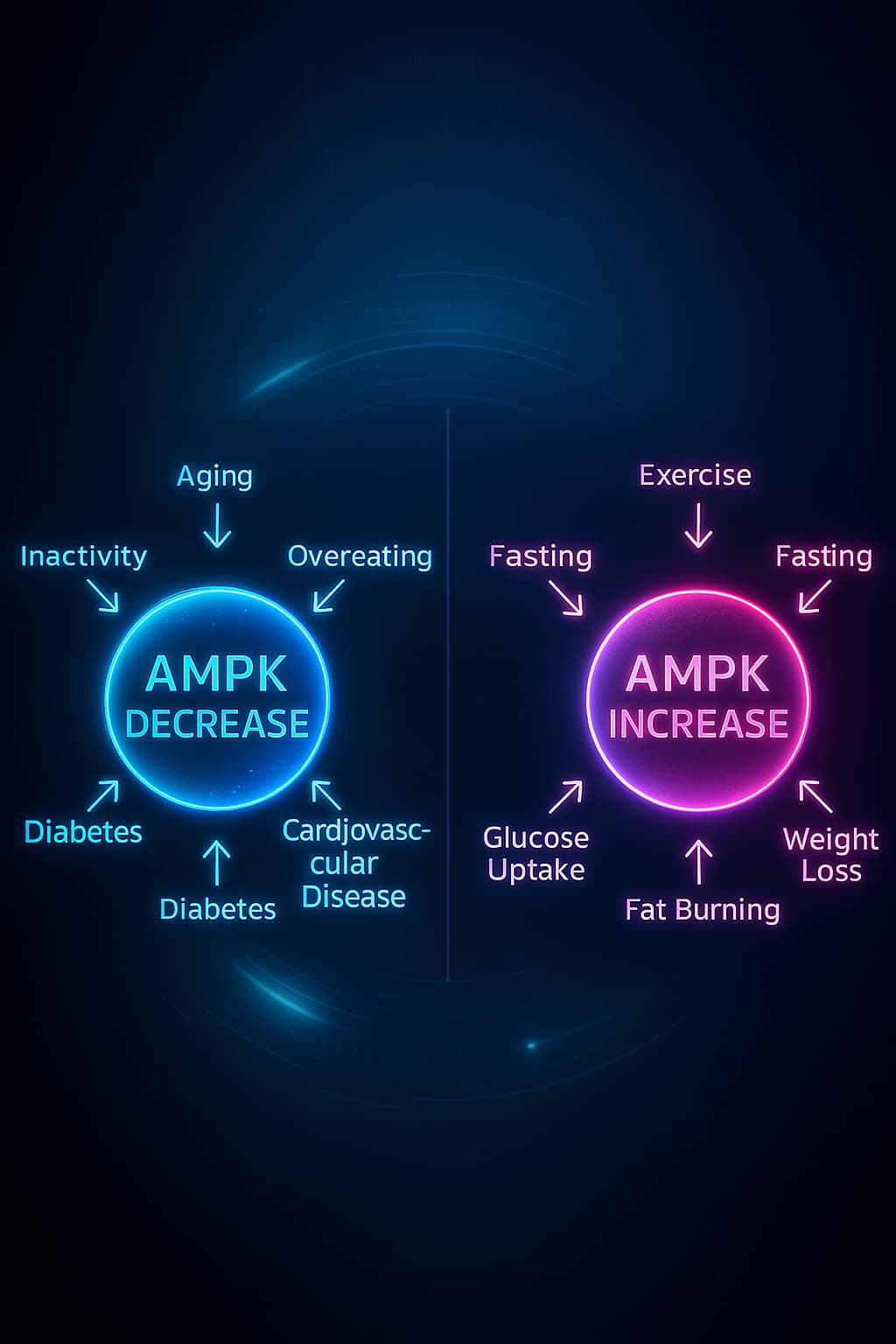
Without AMPK, your cells lose their ability to pause, repair, and rejuvenate, a critical failure in the nutrient-sensing machinery that undermines healthy aging.
Insulin/IGF-1: The Fuel Gauge That Stops Responding
Insulin is like the traffic cop for sugar. Right after a meal, especially one with carbs ,your blood sugar rises, and insulin is secreted from your pancreas. Its job? Direct that sugar into your cells so it can be used for energy or stored for later. It also tells your body, “We’re good on fuel, time to grow and build.”
IGF-1 (Insulin-like Growth Factor 1) works in tandem, especially when protein is around. It promotes cell growth, regeneration, and repair, kind of like your body’s construction foreman. IGF-1 is essential during development and healing, but it also keeps the “growth mode” dial turned up.
But with age and too many snacks, sugars, and sedentary days, this duo starts to malfunction. Your cells stop “hearing” insulin’s knock. Sugar lingers in your bloodstream, inflammation creeps in, and the repair-and-balance signal from IGF-1 gets drowned out. This is how nutrient sensing and aging become tangled, your once-efficient fuel system turns clumsy and confused.
Why?
This breakdown in insulin signaling is a hallmark of deregulated nutrient sensing, driving metabolic disease and aging-related decline.
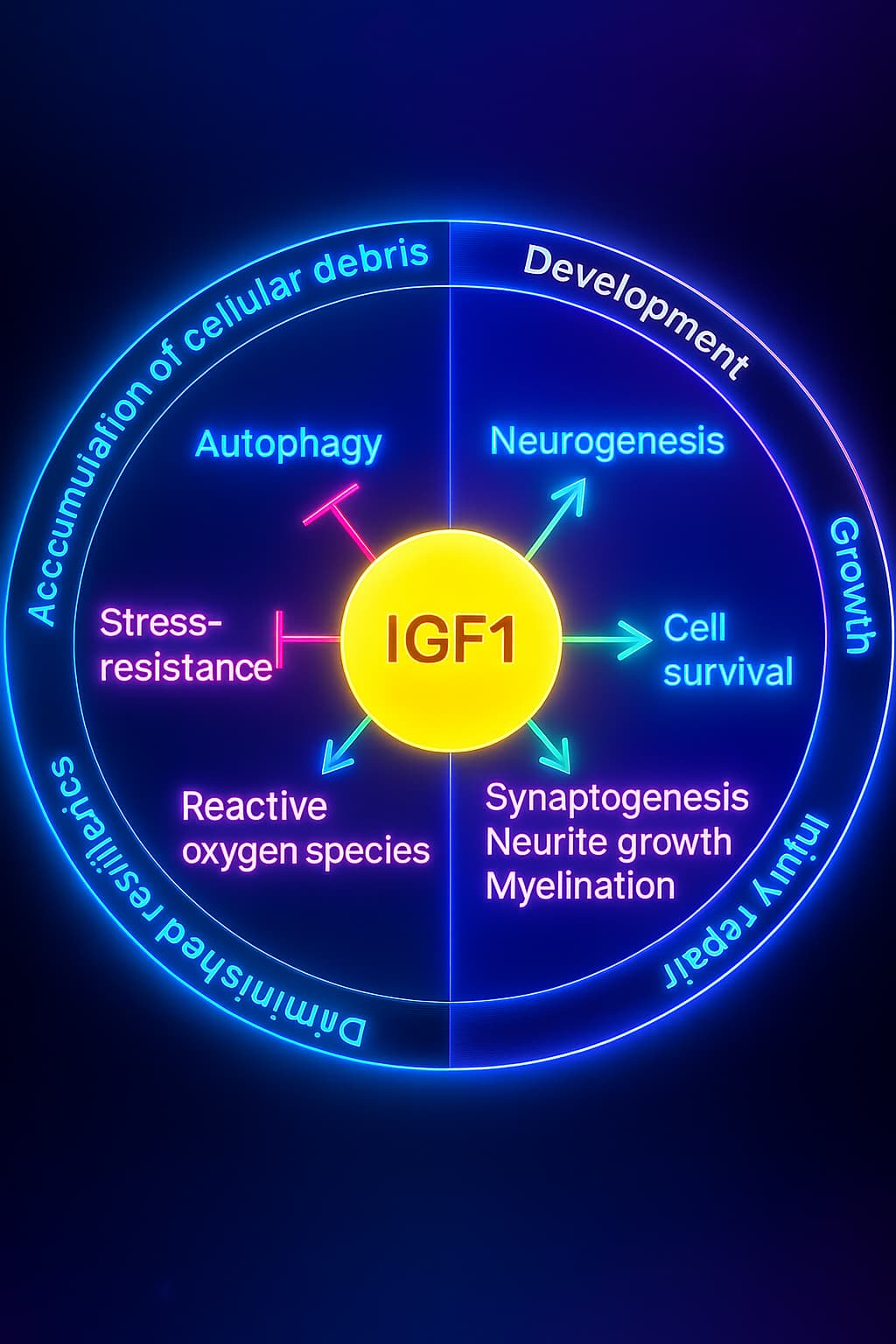
Together, mTOR, AMPK, and insulin/IGF-1 form the triad of your nutrient-sensing system a central control panel for managing growth, repair, and energy. When these switches lose their precision, your body gets stuck in the wrong gear: growing when it should be repairing, storing when it should be cleaning.
This miscommunication is a driving force behind multiple hallmarks of aging and a key target for restoring metabolic flexibility and longevity as we grow older.
Deregulated nutrient sensing doesn’t strike all at once, it’s a slow, layered unraveling tied to both time and lifestyle. On one side, the biology of aging chips away at your cells’ ability to sense nutrients, shift between growth and repair, and maintain balance. On the other, daily habits quietly accelerate the damage.
And together? They create one of the most important hallmarks of aging and one of the biggest threats to autophagy and longevity.
Let’s unpack how this happens, from the inside out.
How Lifestyle Habits Accelerate the Damage
While time erodes the system, modern life pushes it over the edge.
From dawn to dusk, your daily habits shape whether your body is stuck in “growth mode” or has a chance to enter “repair mode.” The wrong patterns supercharge deregulated nutrient sensing and shut down your body’s ability to clean house.
1. Constant Eating and Snacking
Frequent meals keep mTOR chronically active and autophagy suppressed leaving little time for repair and cleanup.
2. Physical Inactivity
Without movement, AMPK stays quiet, and the cellular cleanup crew never gets the memo.
3. Poor or Fragmented Sleep
Your circadian rhythm governs nutrient sensing and mitochondrial function and bad sleep throws the whole system off.
4. High-Sugar, Ultra-Processed Diets
Refined carbs and junk food overstimulate insulin pathways, driving insulin resistance a hallmark of deregulated nutrient sensing.
5. Chronic Stress
Elevated cortisol dulls your cells’ ability to respond to repair cues, keeping autophagy suppressed and inflammation high.
6. Excess Protein or Caloric Intake
More isn’t always better, constant high-protein intake locks mTOR into overdrive, blocking recovery and fueling the decline in nutrient sensing and aging.
The Good News: You Can Rewire the Signals
Deregulated nutrient sensing may be a hallmark of aging, but it’s one of the most fixable. With smart tweaks to your lifestyle, fasting, movement, quality sleep, and nutrient-dense food, you can restore your body’s ability to repair, recycle, and adapt.
Because aging isn’t just about getting older, it’s about how well your cells listen, respond, and renew.
In a healthy, youthful body, nutrient-sensing pathways operate like a finely tuned dashboard. Your cells are like smart engines, constantly scanning for energy availability, nutrient status, and cellular stress. This nutrient-sensing network helps decide when to hit the gas for growth, when to brake for repair, and when to idle for rest.
But as we age, this once-precise dashboard begins to glitch, a phenomenon known as deregulated nutrient sensing, one of the core hallmarks of aging.
The internal signals become scrambled.
The “check engine” light stays off.
And the system gets stuck in growth mode, even when energy levels are critically low.
Breakdown in nutrient sensing leads to widespread metabolic inflexibility:
Nutrient sensing confusion triggers a domino effect:
The result is a runaway system, where aging isn’t just the slow ticking of time, but a self-reinforcing spiral of cellular confusion and breakdown.
This is the hidden cost of deregulated nutrient sensing: not just lost energy balance, but a full collapse of your cell’s ability to adapt, clean up, and survive.
The good news? While nutrient sensing and aging go hand in hand, this is one of the most reversible hallmarks of aging. These science-backed habits can help you reclaim metabolic flexibility and longevity by resetting your internal signals:
1. Embrace Time-Restricted Eating or Intermittent Fasting
Fasting gives your cells space to repair. When you delay or restrict meals (like with a 16:8 or 14:10 window), insulin drops, AMPK activates, and mTOR quiets down. This shift supports autophagy and aging resilience and helps break the cycle of chronic growth signaling.
2. Move With Purpose, Especially HIIT and Strength Training
Physical activity, especially high-intensity interval training (HIIT) and resistance workouts, boosts AMPK, increases insulin sensitivity, and revives your nutrient-sensing pathways. Plus, exercise supports mitochondrial biogenesis, energizing your cells at their core.
3. Cut Back on Sugar and Refined Carbs
Processed foods spike insulin, confuse your cellular dashboard, and overload the insulin/IGF-1 axis. Switching to whole foods with stable energy delivery helps reestablish nutrient balance and minimizes inflammaging, a hallmark tied to autophagy decline.
4. Optimize Sleep for Metabolic Repair
Sleep isn’t just rest, it’s regulation. Deep, regular sleep resets your circadian rhythm, balances AMPK activity, and strengthens your cell’s ability to toggle between growth and repair. Aim for 7–9 hours in rhythm with your body clock.
5. Use Hormetic Stress, Heat, Cold, and Polyphenols
Short bursts of stress, like cold plunges, sauna therapy, or compounds in green tea, berries, and turmeric, gently challenge your cells and activate repair pathways like AMPK and autophagy. This process, called hormesis, improves long-term resilience.
Certain compounds are gaining attention for their ability to modulate nutrient sensing and longevity pathways. While not magic bullets, these supplements can support your body’s effort to rebalance energy signals and promote autophagy and aging defense:
1. Berberine
A plant alkaloid that mimics fasting by activating AMPK and improving glucose metabolism. It’s especially promising in restoring insulin sensitivity.
2. Resveratrol
Found in red grapes and berries, resveratrol inhibits mTOR and boosts AMPK. It mimics calorie restriction benefits and may support metabolic flexibility as we age.
3. Metformin
Originally a diabetes medication, metformin is now being explored for its anti-aging potential. It lowers blood glucose, improves nutrient sensing, and enhances autophagy. but should only be used under medical supervision.
4. Spermidine
This naturally occurring polyamine (found in wheat germ, aged cheese, and soy) stimulates autophagy, modulates mTOR, and has shown lifespan-extending effects in multiple models.
5. PQQ and CoQ10
These mitochondrial boosters help cells create and maintain energy. By supporting mitochondrial function, they indirectly reinforce healthy nutrient sensing and reduce oxidative damage.
Not medical advice. Always check with your doctor before using any supplement.
Beyond diet and exercise, researchers are developing precise tools to rebalance your cells’ nutrient-sensing machinery, unlocking new potential to slow aging at its root.
Some of the most promising strategies:
mTOR Inhibitors (Rapamycin, Rapalogs)
Rapamycin reduces chronic mTOR activation, the “gas pedal” for growth, helping cells switch into repair mode. In animal studies, it extends lifespan, enhances autophagy, and delays age-related diseases. Newer rapalogs aim to deliver these benefits with fewer side effects.
AMPK Activators (Metformin, Berberine)
AMPK is your cellular “emergency brake”, triggered during low energy to boost repair and mitochondrial renewal. Drugs like metformin and natural compounds like berberine help keep this pathway active as we age, improving metabolic resilience and reducing inflammation.
IGF-1 Modulators
Lowering overactive insulin/IGF-1 signaling, common in aging and modern diets, may reduce cancer risk and support longevity. Modulating this pathway promotes balance between growth and maintenance.
Caloric Restriction Mimetics (CR Mimetics)
Compounds like resveratrol, spermidine, and NAD+ boosters mimic the effects of fasting without cutting calories, activating AMPK, inhibiting mTOR, and supporting autophagy and stress resistance.
These interventions are already in clinical trials and biotech pipelines, aiming not just to add years to life, but life to your years.
Your cells may not track macros or count calories, but they are constantly sensing nutrients, energy, and stress. This internal radar system, known as nutrient sensing, helps your body decide when to grow, when to rest, and when to initiate repair. It’s one of the most elegant systems in biology and one of the most crucial for longevity.
But as you age, this harmony begins to break down.
Deregulated nutrient sensing, now recognized as a hallmark of aging, disrupts the delicate balance between growth and repair. The molecular switches that once helped your cells adapt and renew, mTOR, AMPK, and insulin/IGF-1, begin to misfire. Instead of flexible, responsive signaling, your body gets stuck in chronic growth mode. The results? Autophagy declines, metabolic flexibility disappears, and age-related issues start to pile up.
This breakdown doesn’t happen overnight. It’s driven by deep biological changes like DNA damage, telomere shortening, and mitochondrial dysfunction, but it’s also accelerated by modern habits: constant eating, lack of exercise, poor sleep, and stress.
Left unchecked, deregulated nutrient sensing can fuel inflammaging, insulin resistance, stem cell exhaustion, and even cancer. But the the good news it that unlike many other hallmarks, this one is surprisingly reversible.
By embracing intermittent fasting, movement, circadian-aligned sleep, and nutrient-dense eating, you can re-tune your cellular dashboard. Even better? Cutting-edge supplements like berberine, resveratrol, spermidine, and metformin offer powerful tools to support autophagy and aging resilience at the molecular level.
Because aging isn’t just a countdown, it’s a conversation between your cells and their environment. And with the right signals, you can help them keep listening, adapting, and thriving for years to come.
Now that you’ve unlocked the science behind Deregulated nutrient sensing, why stop here? The aging process is a mosaic and Deregulated nutrient sensing is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?