Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“When your cells stop taking out the trash, things get messy, fast.“
Welcome, explorer of life’s hidden machinery.
If you have been following this journey, you already know that aging is not simply about gray hairs or slower recovery. It is about the gradual unraveling of systems deep inside your cells. Today we turn to one of the most essential and often underestimated hallmarks of aging: impaired autophagy. This is the slow decline in your cells’ ability to recycle damaged components and clear out intracellular waste, a process that is vital for maintaining cellular health and resilience.
Autophagy and aging are deeply connected. Autophagy is your body’s built-in recycling and repair network, dismantling broken mitochondria, refolding or removing misfolded proteins, and clearing faulty organelles before they can cause harm. When this process falters, the result is toxic buildup, chronic inflammation, reduced energy production, and widespread dysfunction. These changes do not stay isolated within a single cell; they ripple outward, fueling neurodegeneration, tissue decline, and the loss of physical vitality.
In this exploration, we will uncover:
To understand autophagy and aging is to recognize the importance of keeping the cell’s inner world clean and functional. By supporting this process, we give our cells the chance to maintain their energy, clarity, and balance for longer.
Let us open the door to the cell’s recycling center and see what it takes to keep the cleanup crews working at their best.
Your cells are constantly working in building, repairing, and recycling. But as you age, this balance tips. Proteins misfold, mitochondria falter, and waste starts piling up. Left unchecked, this intracellular clutter becomes toxic, fueling inflammation, dysfunction, and aging.
That’s where autophagy comes in, your body’s internal recycling system.
Autophagy (Greek for “self-eating”) identifies damaged proteins, defective organelles, and worn-out cellular parts. These are wrapped in autophagosomes, delivered to lysosomes, and broken down into usable fuel like amino acids, fats, and sugars.
In short: Autophagy turns cellular trash into clean energy, keeping your cells resilient, clear, and youthful.
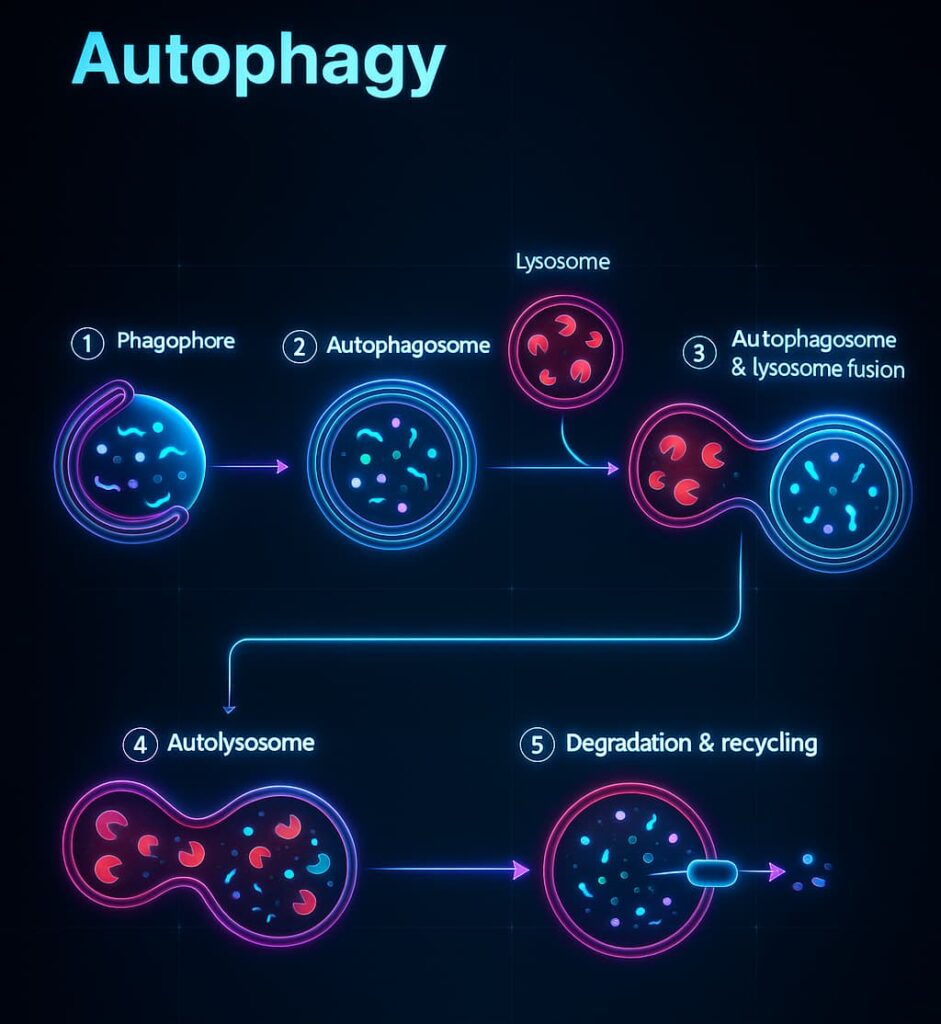
Autophagy is also a stress response system. It powers survival during fasting, clears damage during infection, and adapts cells to stress. When autophagy flows, your cells stay efficient and energized.
But when it breaks down?
You get impaired autophagy: a key hallmark of aging linked to neurodegeneration, cancer, and metabolic decline.
That’s why supporting autophagy is so essential to healthy aging and why autophagy dysfunction is more than a symptom. It’s a driving force behind cellular decline.
Autophagy is one of your body’s most powerful internal repair systems. In youth, it functions like a state-of-the-art recycling plant: fast, responsive, and always on call.
But as the years go by, this cellular cleanup system begins to falter.
The recycling crew misses shifts. The junk starts piling up. And what was once a flawless defense against intracellular waste becomes a sluggish, error-prone process.
So what exactly causes impaired autophagy with age?
1. Growth and Cleanup Fall Out of Balance: mTOR vs. AMPK
Inside every cell, growth and repair run on separate tracks and two major signals manage that switch: mTOR and AMPK.
In youth, these signals stay in sync.
But with aging (and modern habits like constant snacking and sedentary living), mTOR stays overactive, and AMPK declines, a classic example of deregulated nutrient sensing, another key hallmark of aging..
The result? Cells keep trying to grow even when they should clean.
Autophagy gets suppressed, and cellular cleanup decline begins.
2. Fewer Supplies to Build Autophagosomes
Autophagosomes, the cleanup vesicles that wrap waste, need lipids, proteins, and enzymes to form.
But with age, the production of these materials drops – in part due to epigenetic alterations that reduce autophagy-related gene expression and weaken the transcription of key repair proteins:
It’s like trying to clean house with no trash bags left.
3. Lysosomes Lose Their Digestive Power
Lysosomes, the acidic compartments that degrade waste, also deteriorate with age:
Part of this is due to oxidative stress often worsened by mitochondrial dysfunction. But another culprit is lipofuscin (a byproduct of proteostasis loss) a pigment-like waste that builds up in lysosomes and clogs the system.
Think of it like gunk in your kitchen drain: flow slows, and nothing gets processed properly.
4. The Conveyor Belt Stalls: Impaired Autophagic Flux
Autophagy isn’t one action. it’s a cycle.
But in older cells, the flow breaks down. Even if autophagosomes form, they may not reach lysosomes or get degraded.
This stalled process is known as impaired autophagic flux and it’s one reason aging and intracellular waste go hand in hand, especially as altered intercellular communication disrupts the signaling required to keep this cycle moving..
Imagine garbage bags piling up at the door, never making it to the recycling center.
5. Mitophagy Fails: Broken Mitochondria Pile Up
Healthy mitochondria power your cells. Damaged ones leak toxins and trigger inflammation.
Normally, cells remove them through mitophagy (targeted form of autophagy).
But with age, the tagging system breaks, and the machinery grows weak.
Result? Old, toxic mitochondria linger. releasing ROS, damaging DNA, and accelerating the aging process.
6. Autophagy Stops Responding to Stress
One of autophagy’s strengths is its adaptability.
Fasting, exercise, or infection usually trigger a surge in activity.
But aging blunts that response.
Cells become resistant to signals that should kick autophagy into gear.
Why? A mix of chronic inflammation, epigenetic alterations, and altered intercellular communication all of which interfere with the cell’s ability to detect and respond to stress.
Instead of adapting, cells stagnate, allowing damage to build.
The Big Picture: A System-Wide Breakdown
As we age, autophagy and aging collide in a cascade of dysfunction:
This autophagy dysfunction in aging doesn’t just follow aging, it drives it.
Clearing out waste becomes harder.
Damaged mitochondria stay online.
And the systems meant to keep cells young begin contributing to their decline.
Autophagy and aging are tightly linked and when this vital cleanup system fails, the consequences go far beyond a bit of mess. It becomes a full-blown biological hazard.
Instead of recycling and renewal, cells get buried under layers of broken parts and intracellular waste. Impaired autophagy disrupts every system inside the cell and over time, the damage ripples outward. Sometimes, cells become so overwhelmed that they enter cellular senescence, a dysfunctional state where they stop dividing and start secreting harmful signals.
1. Toxic Buildup: Junk Turns Hostile
In healthy cells, autophagy clears out misfolded proteins, oxidized lipids, and defective mitochondria.
But when autophagy slows, these toxic components accumulate:
And this mess doesn’t just sit quietly. It fuels inflammation, drains your resilience, and accelerates aging from the inside out, just like what we see with proteostasis loss and mitochondrial dysfunction.
2. Energy Shortages: Power Grid Instability
One of autophagy’s hidden superpowers? It recycles broken-down parts into fuel, especially during stress, fasting, or energy shortfalls.
When this cellular cleanup system declines, your cells lose the ability to adapt:
This kind of energy failure doesn’t just make you feel tired, it feeds directly into mitochondrial dysfunction and can even wear down your stem cells, leaving them too depleted to repair or renew your tissues.
3. Fragile Stress Response: Resilience Fades
Autophagy is like an internal first responder.
When something goes wrong, infection, injury, or oxidative stress, it’s supposed to kick into high gear and clear the damage.
But as you age, that response weakens. Your cells struggle to adapt.
They can’t deal with oxidative stress (especially when mitochondria are leaky), and they lose the ability to shift gears when conditions change.
And why does that happen? A mix of deeper aging changes like epigenetic alterations that silence key cleanup genes, and deregulated nutrient sensing, which confuses your cell’s internal dashboard.
4. Chronic Inflammation: The Rise of Inflammaging
When cellular waste isn’t cleared, your immune system notices, even without infection.
This constant low-level alarm sparks inflammaging: a chronic, smoldering inflammation now recognized as a root cause of many age-related diseases, including:
This immune overactivation damages healthy tissues and accelerates aging and it’s made worse by altered intercellular communication, another key hallmark of aging that disrupts how cells coordinate and calm inflammation.
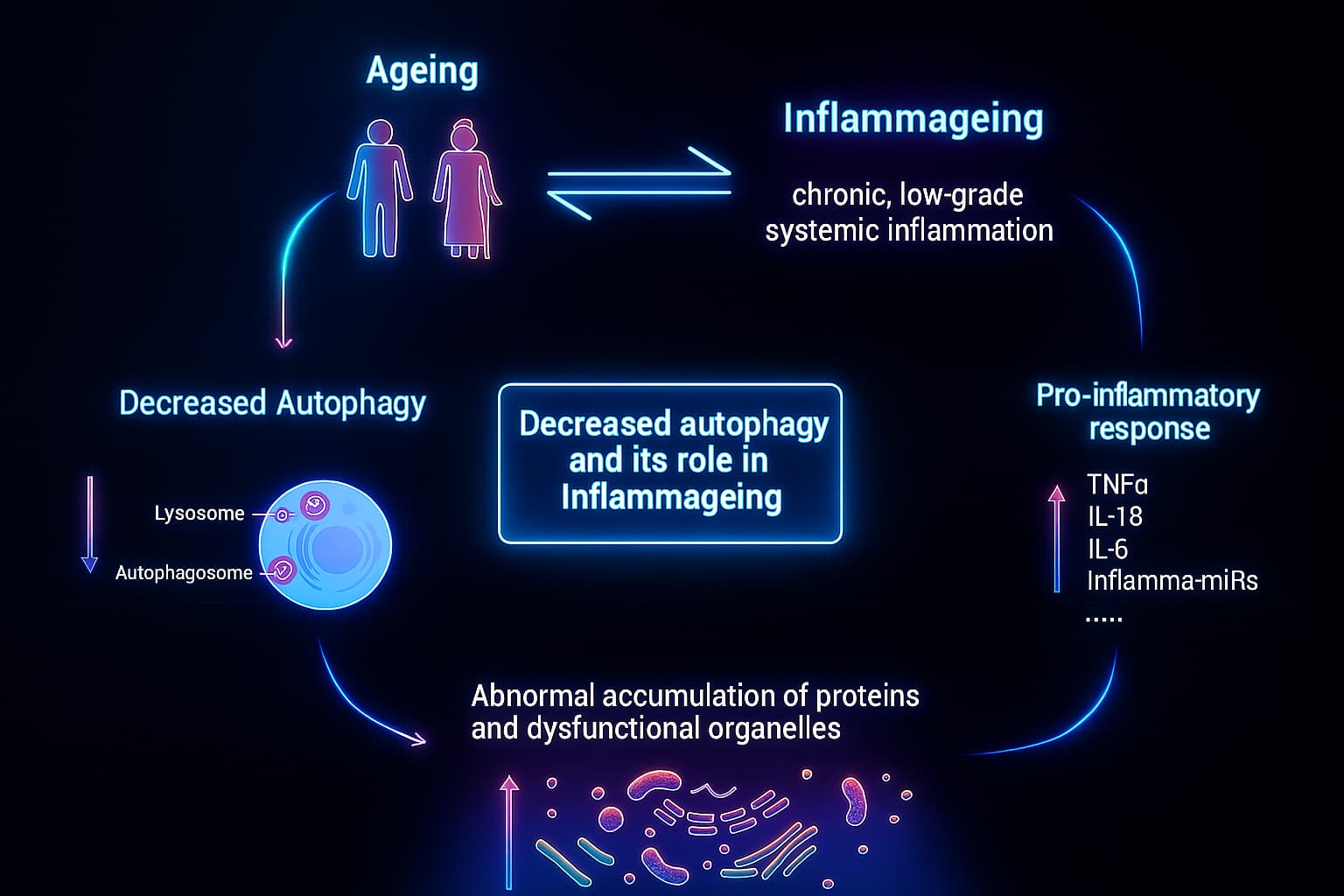
Once autophagy declines, the whole cell begins to unravel:
This isn’t just a symptom. it’s a core driver of aging.
Autophagy dysfunction in aging weakens your cells’ ability to adapt, renew, and survive.
It turns resilience into fragility.
And it’s why protecting your cellular cleanup systems is one of the most powerful strategies for long-term health.
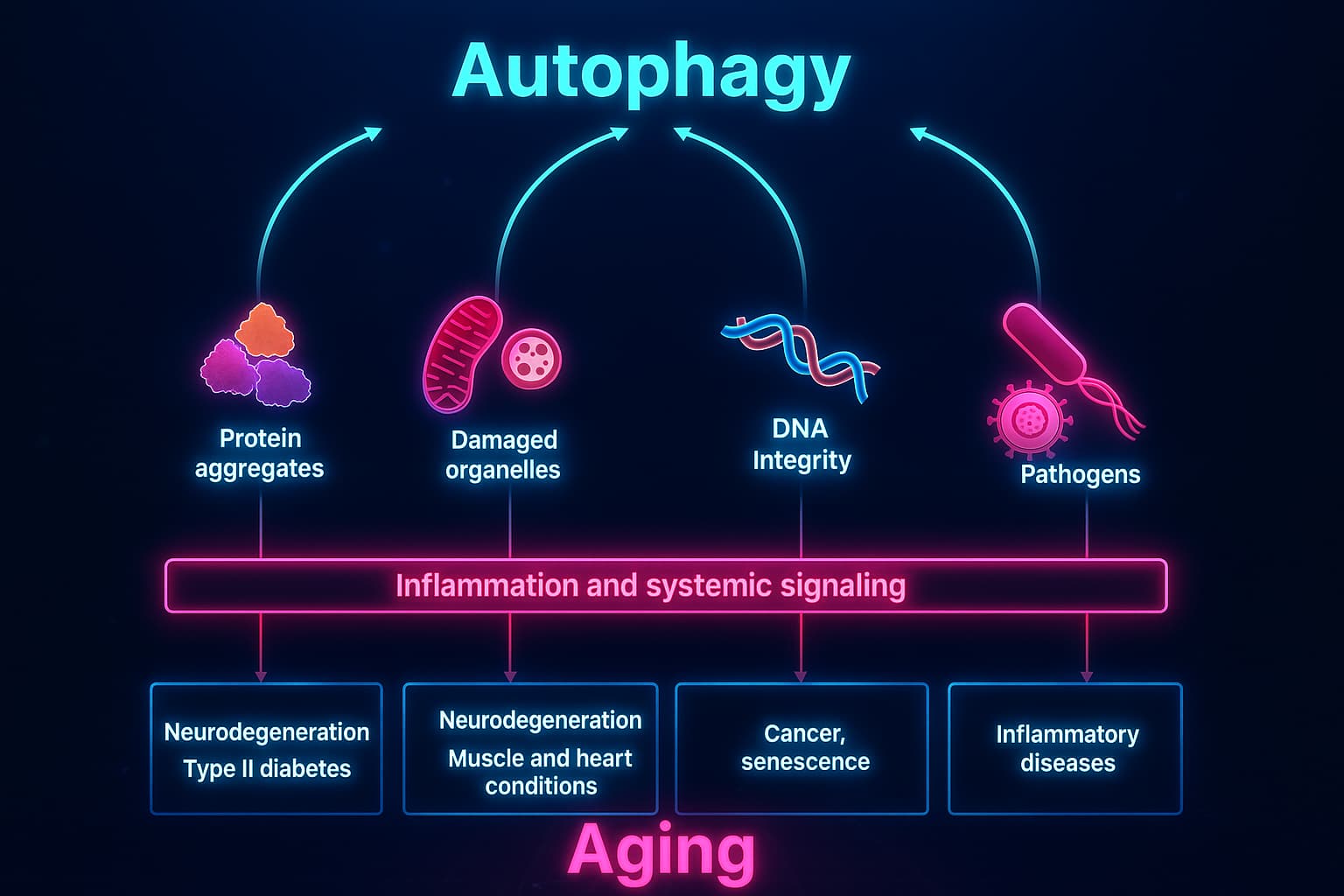
Absolutely and you don’t need a lab coat or a prescription.
One of the most exciting things about autophagy and aging is this: you can influence it.
Yes, aging slows down your cell’s cleanup crew, but you’re not powerless. In fact, your everyday choices play a huge role in keeping your intracellular recycling system alive and well.
Certain habits, especially those that mimic mild cellular stress, can turn on autophagy naturally. And a few supplements may give it a gentle nudge.
Let’s break it down:
1. Fasting & time-restricted eating
Fasting, or even just compressing your eating window into 8–10 hours per day, is one of the most potent natural triggers of autophagy.
Why? When nutrients are scarce, your body shifts from “build mode” to “repair mode.” That’s when AMPK is activated, mTOR is dialed down, and autophagy kicks into gear.
Think of fasting as pressing the reset button for your cells.
They pause the growth projects and start sorting, recycling, and repairing instead.
Best practices:
Try 14–16 hour fasts a few times a week, or experiment with time-restricted eating (like 10am–6pm). It doesn’t have to be extreme, consistency matters more than length.
2. Regular exercise
Exercise, especially aerobic or resistance training, stimulates autophagy in muscles, liver, fat tissue, and even the brain. It helps clear out broken mitochondria, reduce oxidative stress, and keep your energy systems nimble.
It’s like calling in a mobile cleanup crew after a big event, everything gets tidied up and tuned.
3. Sleep
Autophagy naturally follows a circadian rhythm and it tends to peak during sleep, especially in the brain.
While you’re resting, your body does deep maintenance:
But poor sleep shuts that whole process down. Missed sleep = missed recycling.
Tip: Prioritize 7–8 hours of high-quality sleep with minimal light exposure and consistent timing to keep your autophagy rhythms on track.
4. Hormetic stress: challenge creates strength
Your cells thrive when pushed just a little out of their comfort zone.
This idea is called hormesis, the principle that low-level stress activates protective pathways like autophagy.
How to apply it:
Think of hormesis like a workout for your cells, it leaves them stronger, cleaner, and more adaptable.
Lifestyle is the foundation but certain natural compounds may offer extra support for your cellular recycling system, especially as you age.
1. Spermidine
Found in wheat germ, soy, and fermented foods, spermidine is one of the most promising autophagy activators in longevity science. It helps trigger cellular cleanup and has been linked to improved mitochondrial quality control and even extended lifespan in animal models.
2. Resveratrol
This compound, found in red grapes, berries, and peanuts, activates AMPK and inhibits mTOR, making it a natural autophagy booster. It also reduces inflammation and supports metabolic health.
3. Curcumin
From turmeric, curcumin modulates cellular stress pathways, supports autophagy, and reduces chronic inflammation, making it a favorite in anti-aging and neuroprotection research.
4. Berberine
This plant alkaloid mimics the effects of fasting by activating AMPK. It’s been shown to improve glucose metabolism, support healthy weight, and stimulate autophagy.
5. Urolithin A
Derived from pomegranate compounds, Urolithin A enhances mitophagy helping cells identify and recycle damaged mitochondria. It’s currently being studied in human trials for improving muscle endurance and cellular energy with age.
Not medical advice. Always check with your doctor before using any supplement.
Keep in mind:
These supplements aren’t magic pills, but they can offer meaningful support when combined with healthy habits.
Like good tools in a well-run workshop, they help your cells stay clean, adaptable, and resilient.
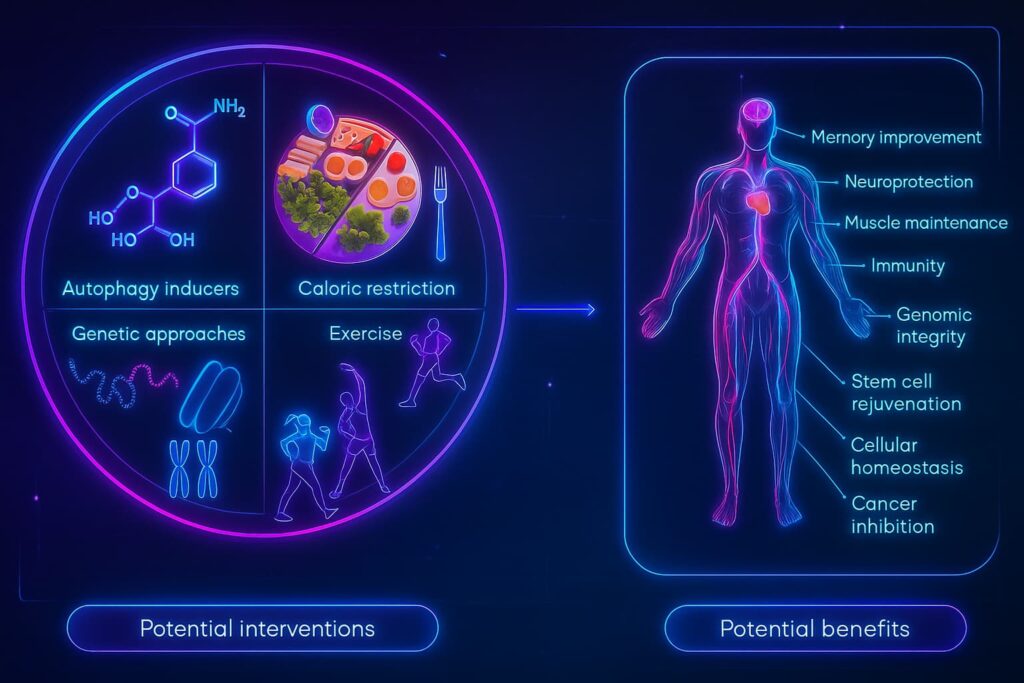
New frontiers in cellular recyclinWhile lifestyle and nutrition can support your cellular cleanup system, researchers are going further, asking a bold question:
Can we flip the switch on impaired autophagy directly, especially in aging cells?
The answer is increasingly yes. And it’s opening up one of the most exciting frontiers in longevity science.
Scientists are developing therapies that aim to reactivate autophagy, restore mitochondrial quality control, and reverse autophagy dysfunction in aging. These strategies don’t just treat symptoms, they target the root causes of cellular aging by enhancing waste clearance and cellular resilience.
Some of the most promising breakthroughs under development:
1. Rapamycin and mTOR Inhibition
Discovered in soil bacteria from Easter Island, rapamycin works by inhibiting mTOR, the molecule that normally keeps cells focused on growth and protein synthesis.
By dialing down mTOR, rapamycin shifts the cell from growth mode to repair mode, allowing autophagy to resume.
In animal studies, rapamycin has:
However, long-term use can have side effects, which is why newer versions, known as rapalogs, are in development as safer alternatives for humans.
2. Caloric Restriction Mimetics: Fasting Without the Fast
Caloric restriction (CR), eating fewer calories without malnutrition, is one of the most proven ways to activate autophagy and extend lifespan.
But not everyone wants to cut 30% of their food intake.
That’s where caloric restriction mimetics come in: compounds that mimic the effects of fasting without actually reducing calories.
Examples include:
These compounds are under intense study, not just for diabetes, but for reversing cellular cleanup decline and slowing biological aging.
3. Gene Therapy: Fixing Autophagy at the Source
One of the most exciting frontiers? Using gene therapy to directly enhance the autophagy machinery in aging cells.
Researchers are exploring ways to:
These strategies could reboot the cellular recycling system, especially in diseases where aging and intracellular waste play a central role, like Alzheimer’s, Parkinson’s, and sarcopenia.
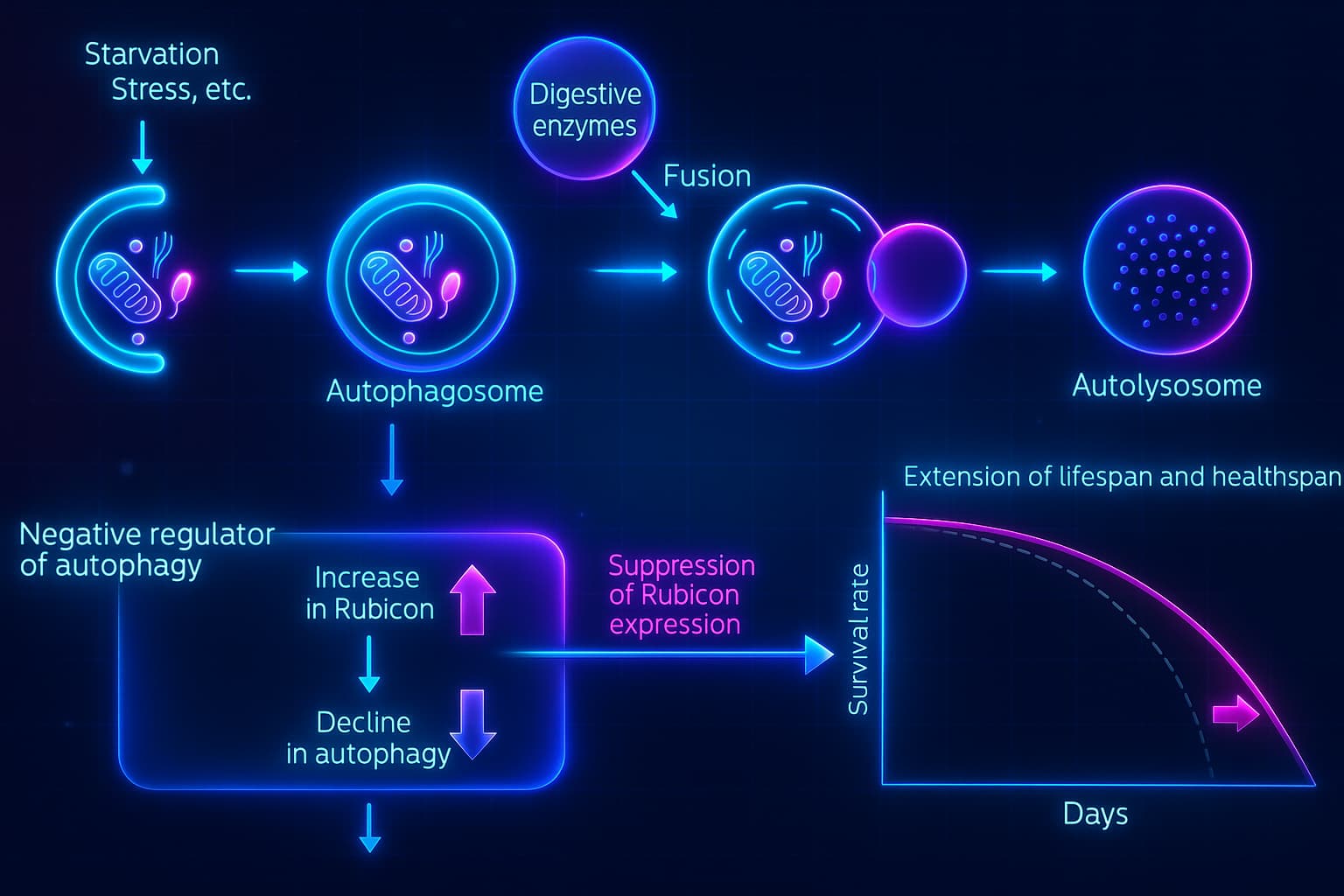
A Glimpse Into the Future
These breakthroughs represent more than hope, they mark a turning point in how we approach aging.
Instead of patching up the damage, we’re learning how to restore the body’s own repair systems ,with autophagy at the center.
Imagine a future where we clear toxic buildup, support mitochondrial renewal, and slow aging, not with magic pills, but by reactivating the ancient survival systems already inside you.
The science is early. but the shift is real.
So there you have it, longevity explorer, a deep dive into one of the most powerful and underrated forces behind healthy aging: autophagy.
We’ve seen how impaired autophagy isn’t just a side effect of getting older, it’s a key hallmark of aging in its own right. When this essential cellular cleanup system slows down, your cells start to drown in their own debris: broken mitochondria, misfolded proteins, and oxidized waste. The result? Chronic inflammation, energy loss, and a ripple of dysfunction across your entire body.
That’s why the link between autophagy and aging is so important and why restoring this ancient survival system could be a game-changer for longevity.
The good news? You can support your autophagy starting today.
From simple lifestyle shifts like fasting, exercise, and deep sleep, to powerful autophagy supplements like spermidine, resveratrol, berberine, and Urolithin A, your daily habits can reignite your cell’s internal recycling team and help reverse the tide of cellular cleanup decline.
Even more exciting? Scientists are pushing the frontier further with therapies designed to reboot autophagy at its source, using tools like mTOR inhibitors, caloric restriction mimetics, and even gene therapy to enhance repair and resilience at the cellular level.
Autophagy isn’t just about clearing waste, it’s about protecting your long-term vitality.
So keep your cleanup crew strong, your mitochondria fresh, and your cellular systems agile, because when autophagy flows, longevity follows.
Now that you’ve unlocked the science behind Autophagy , why stop here? The aging process is a mosaic and Autophagy is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?