Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

If you thought aging science was only about eating less or jogging more, 2025 is here to surprise you. This year has delivered true longevity breakthroughs that sound like science fiction but are grounded in real biology. The focus is shifting from surface-level health advice to tackling the hallmarks of aging directly. Studies are showing how telomere extension can protect against telomere shortening, how stem cell therapy can counteract stem cell exhaustion, and how precise genetic tuning can restore regeneration without triggering cancer. Other hallmarks like genomic instability, chronic inflammation, and cellular senescence are also being addressed in new and creative ways, bringing the dream of slowing biological aging closer than ever.
These are not just incremental steps. They are bold anti-aging breakthroughs, reshaping our understanding of how long and how well we can live. The field of regenerative medicine is advancing faster than ever, revealing strategies to keep our tissues youthful, our repair systems active, and our immune defenses strong. From the promise of telomere extension to the potential of stem cell therapy, the science of human longevity is rapidly moving from theory to reality.
At the ends of every chromosome sit the telomeres, repetitive DNA sequences that protect our genetic material. Picture them as the plastic caps on shoelaces that keep the lace from fraying. Each time a cell divides, the telomeres shorten slightly. Eventually, they become too short to protect the DNA, and the cell either stops dividing or slips into dysfunction. This connection between telomere and aging is one of the most established principles in longevity science. Shortened telomeres are strongly linked with weaker immunity, slower wound healing, and age-related decline. The pursuit of telomere extension has therefore become a central theme in every major anti-aging breakthrough.
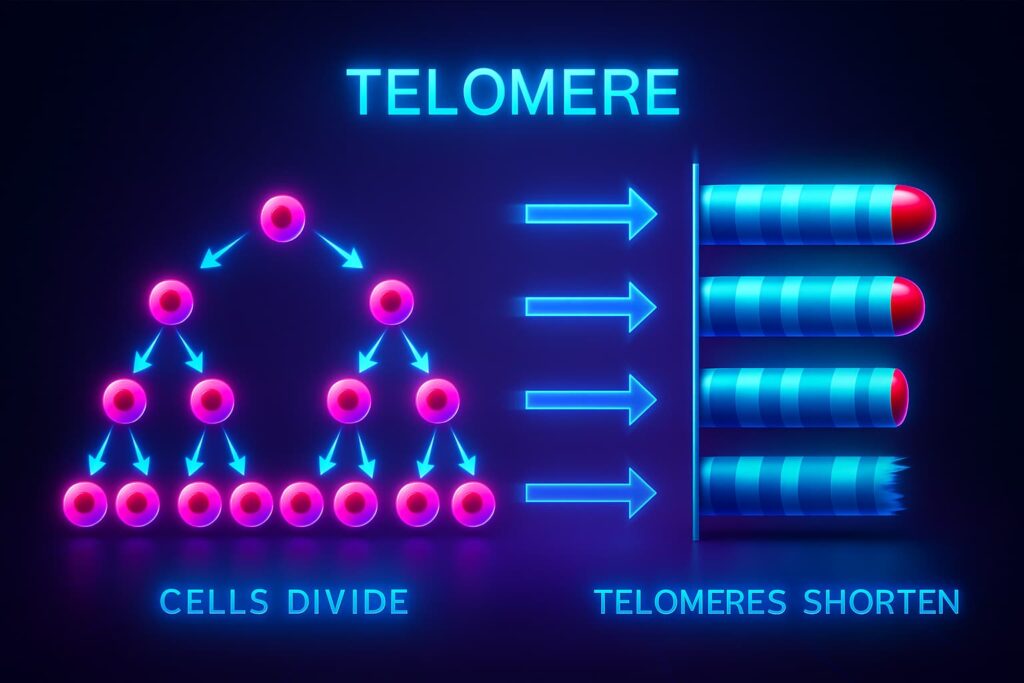
Nature has already invented a tool to keep telomeres long: telomerase, an enzyme that can add DNA back onto chromosome ends. Unfortunately, most of our adult cells produce very little of it. Stem cells make more, but even they eventually lose their regenerative edge as telomeres erode. Scientists have long dreamed of using telomerase therapy to preserve telomeres and delay cellular aging. The challenge has always been safety and precision. Activating telomerase too strongly can fuel uncontrolled growth, while weak activation does not stop the countdown. The dream of regenerative medicine has always been to find the perfect balance that extends telomeres safely.
In 2025, a team at the Chinese Academy of Sciences and Peking University decided to take a smarter approach. Instead of simply overactivating telomerase, they engineered a synthetic telomerase RNA template that improved efficiency while avoiding instability. They tested this innovation in induced pluripotent stem cells (iPSCs) derived from patients. iPSCs are central to stem cell therapy and regenerative medicine because they can develop into many tissue types. The question was bold but clear: could this engineered template deliver a controlled form of telomere extension that kept stem cells youthful without risking damage?
The results were striking. The modified iPSCs with synthetic telomerase RNA divided far beyond their normal lifespan and, importantly, maintained genomic stability. Their DNA remained intact and functional even after repeated replication. Rather than collapsing into dysfunction, the cells preserved their youthful vigor. It was a measured and controlled longevity breakthrough, not a reckless push toward uncontrolled growth.
The survival curve in the figure makes this clear. Cells treated only with TERT (black line) stopped dividing after about 40 doublings, while cells given TERT plus the engineered telomerase RNA (red line) continued dividing to nearly 100 population doublings. This dramatic increase shows the power of telomere extension in delaying cellular aging. It demonstrates how telomerase therapy can enhance the replicative lifespan of stem cells, strengthening the foundation for stem cell therapy and advancing the goals of regenerative medicine.
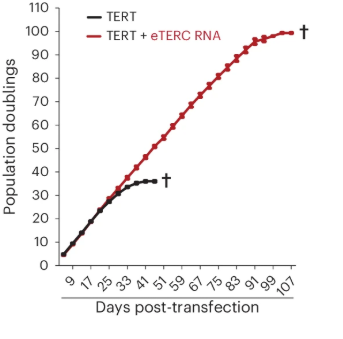
Stem cells are the body’s frontline repair system, replacing lost cells and patching up tissues. But once telomeres shorten, even stem cells lose their spark. This study shows that telomerase therapy could extend the working life of stem cells by reinforcing telomeres and ensuring reliable telomere extension. The implications for stem cell therapy are immense: longer-lasting grafts for the heart or brain, stronger tissue repair, and a new foundation for regenerative medicine.
For the field of aging, this marks a true anti-aging breakthrough. By targeting the link between telomere and aging, scientists are moving beyond passive observation and into active engineering of healthier, longer-lived cells. It is not a promise of immortality, but it is a practical roadmap for longevity breakthroughs that could one day reshape human health and lifespan.
Deep inside the bone marrow lives a population of hematopoietic stem cells, the master builders of the blood and immune system. They continuously produce red blood cells that carry oxygen, white blood cells that fight infections, and platelets that help us heal wounds. With age, however, these cells lose their balance. They divide less often, generate fewer protective immune cells, and lean toward producing inflammatory lineages that accelerate decline. The weakening of hematopoietic stem cells is one reason our immune system becomes fragile and recovery slows as we grow older. Unlocking this process is viewed as a true longevity breakthrough and a core focus of regenerative medicine.
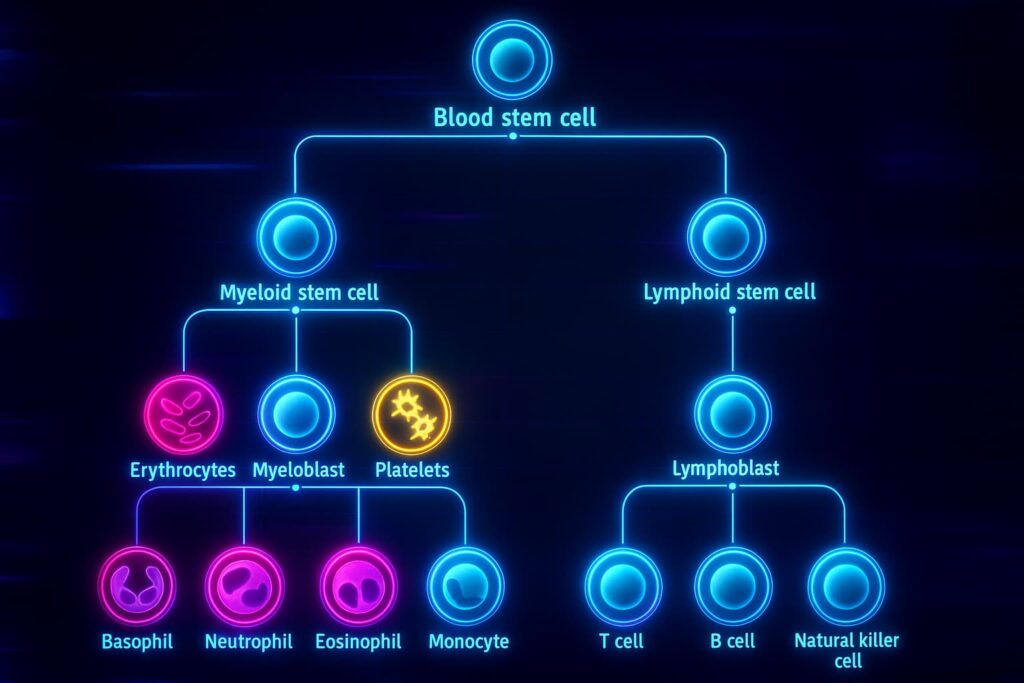
In 2025, scientists at Harvard Medical School and Boston Children’s Hospital asked a bold question. Could the body still hold a hidden reserve of youth within its own marrow? To their surprise, even in older mice they found a rare subset of hematopoietic stem cells that retained youthful qualities. The researchers transplanted these younger-acting cells into middle-aged mice to test whether they could spark true stem cell therapy effects. Their goal was to see if a small biological push could deliver real stem cell rejuvenation and improve systemic health.
The results were remarkable. Mice receiving the youthful hematopoietic stem cells lived about 9 percent longer than untreated animals. They showed stronger muscles, better stamina, and more resilient immune systems. Even more striking, molecular analysis revealed that their epigenetic age shifted in a younger direction. This was not a placebo effect but a measurable, biological anti-aging breakthrough. A small infusion of youthful marrow cells was enough to spark system-wide stem cell rejuvenation and measurable lifespan extension.
The survival curve shown in the figure makes this effect clear. After transplantation (marked by the arrow), the mice that received youthful hematopoietic stem cells (blue line) maintained higher survival probabilities for much longer compared to the controls (gray and gold lines). This demonstrates that targeted stem cell therapy directly improved survival, offering visual proof of a true longevity breakthrough. By boosting resilience at the cellular level, these transplanted cells extended both healthspan and lifespan, confirming the potential of regenerative medicine to restore vitality and slow the aging process.
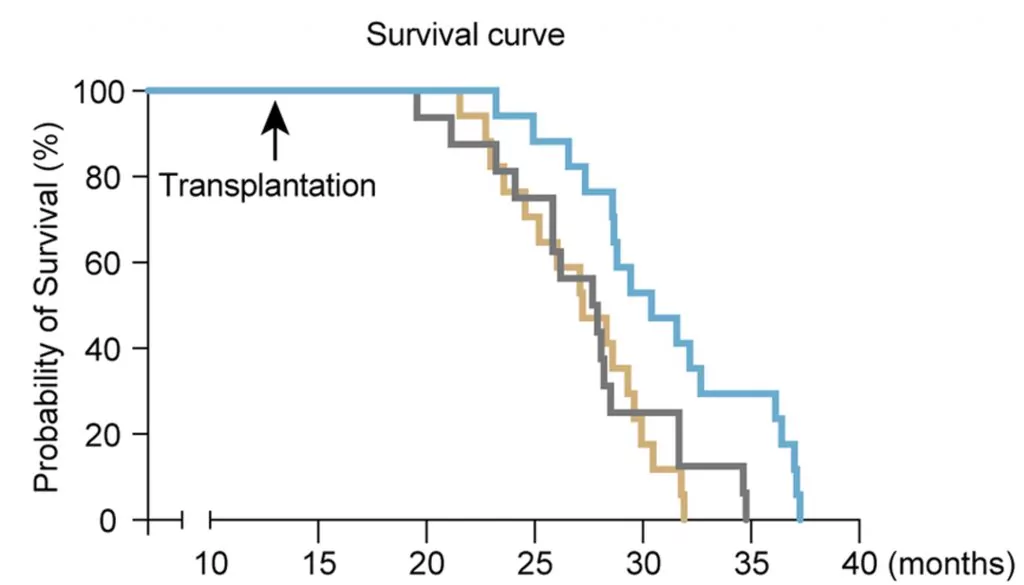
This discovery opens an exciting door for regenerative medicine. If youthful subpopulations of hematopoietic stem cells can be found even in older organisms, then our bodies may already carry the blueprint for their own renewal. Future stem cell therapy could focus on isolating and reactivating these cells to restore immune balance, fight inflammation, and slow the biological processes tied to telomere extension and aging. For anyone chasing longer, healthier lives, this research suggests that true longevity breakthroughs might already be hiding within our own bones, waiting to be awakened.
Cells have their own safety brakes, and one of the most important is the p21 gene. Its role is to stop damaged or stressed cells from dividing. This protects us from cancer but comes at a cost. As telomeres shorten and DNA accumulates damage, p21 activity rises and many cells, including stem cells, are forced into permanent arrest. Over time, this protective mechanism contributes to stem cell exhaustion, reduced tissue repair, and the steady decline of health. For years, scientists thought targeting p21 was too dangerous, yet its central role in regeneration made it an intriguing candidate for a future anti-aging breakthrough. If controlled properly, it could help unlock the next true longevity breakthrough in safe stem cell therapy.
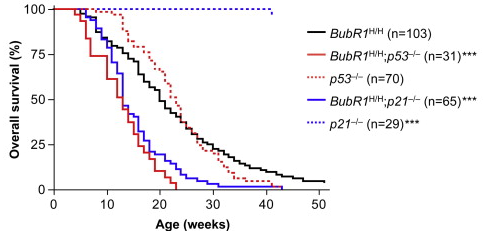
In 2025, a team publishing in Nature Genetics decided to test this hypothesis head-on. They worked with mice whose telomeres were already dysfunctional, a model often used to mimic accelerated aging. By deleting the p21 gene, the researchers asked a daring question: could they restore stem cell activity, improve repair, and achieve reliable lifespan extension? And just as importantly, could they do this without tipping the scales toward cancer?
The outcome was groundbreaking. Removing p21 rejuvenated stem cell activity, improved tissue regeneration, and led to clear lifespan extension in the mice. Crucially, this benefit came without an increase in cancer incidence, overturning one of the longest-standing concerns in the field. The results showed that under precise conditions, releasing this genetic brake did not cause uncontrolled growth but instead unlocked healthy renewal.
The survival curve in the figure illustrates this result. Mice lacking the p21 gene (dotted blue line) and those with telomere dysfunction plus p21 deletion (solid blue line) showed significantly better survival compared to controls (black line). In contrast, deleting p53 (red lines) did not deliver the same benefit and carried higher risks. This visual evidence confirms that targeting p21 produced a safe anti-aging breakthrough, offering clear regenerative medicine potential. By restoring stem cell function without fueling instability, p21 deletion represents a rare and powerful longevity breakthrough where lifespan extension is achieved with safety intact.
This study offers a rare and valuable combination in aging biology: restored stem cell function alongside safe lifespan extension. Most interventions that push regeneration end up raising cancer risk, but here the balance tilted in favor of renewal without instability. For the future of stem cell therapy and regenerative medicine, it signals a path toward carefully adjusting the body’s internal brakes to achieve powerful yet safe results.
For anyone who dreams of not just living longer but living stronger, this work highlights a new frontier. The pursuit of longevity breakthroughs often faces trade-offs, but the p21 study suggests those trade-offs can be overcome. Paired with other strategies like telomere extension and stem cell therapy, it reinforces the vision of a future where anti-aging breakthroughs move from the lab to real-world medicine.
2025 has been a landmark year for the science of aging, marked by true longevity breakthroughs. Across three groundbreaking studies, we have seen that aging is not just a slow and inevitable decline but a process that can be shifted, slowed, and in some cases even reversed.
The first study on telomerase therapy showed how synthetic telomerase RNA can protect the fragile ends of our chromosomes, strengthening the link between telomere and aging. By advancing controlled telomere extension, scientists extended the lifespan of stem cells in the lab and opened the door to regenerative medicine strategies that could keep the body’s repair systems strong for far longer. This represents an anti-aging breakthrough that directly targets DNA protection and renewal.
The second advance came from hematopoietic stem cells, the guardians of blood and immunity. Researchers proved that even in older animals, hidden reserves of youthful cells still exist. By transplanting them, they achieved powerful stem cell therapy effects, delivering measurable stem cell rejuvenation and meaningful lifespan extension in mice. This discovery is more than a technical success; it is a true longevity breakthrough, showing that the body itself may carry the seeds of renewal.
The third paper focused on genetic brakes that limit regeneration. By deleting the p21 gene, scientists restored stem cell activity and achieved safe lifespan extension without triggering cancer. This bold approach showed that regenerative medicine can balance repair and safety. It represents another anti-aging breakthrough, proving that we can carefully tune the body’s genetic switches to achieve renewal without losing stability.
Together, these studies paint a vision for the future of stem cell therapy, regenerative medicine, and telomere extension. They demonstrate that protecting DNA, rejuvenating stem cells, and fine-tuning genes are not science fiction but real strategies now emerging from the lab. At the center of all three lies the promise of safe, effective, and meaningful lifespan extension.
The message is clear: longevity breakthroughs are no longer distant dreams. With anti-aging breakthroughs in telomeres, stem cells, and genetic repair, the science of regenerative medicine is moving us closer to a future where humans can live not only longer but healthier and more vibrant lives.