Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“We are not just the code written in our genes, we are the editors of that code as well.“
Welcome, traveler of the inner code.
If you’ve arrived here, you are standing at the threshold of one of biology’s most elegant mysteries: the epigenome. These are not the genes themselves, but the subtle chemical marks that rest upon them, tiny molecular scribbles that decide which instructions are read and which remain silent.
Think of your DNA as a vast library, containing every chapter of your biological story. The epigenome is the librarian, deciding which books are placed on the desk and which are locked away on high shelves. In youth, the librarian is precise and sure-handed. But with age, the system begins to falter. Pages are misplaced, the wrong books are opened, and the clarity of the story fades. This drift (known as epigenetic alteration) is one of the recognized hallmarks of aging, quietly shaping the way our cells behave and remember who they are.
These changes ripple through your body’s systems, influencing inflammation, tissue repair, energy production, and even the stability of your identity at the cellular level. They are not fixed in stone; they are dynamic, and that is where the promise lies. If the marks can be altered, perhaps they can be restored.
In this exploration, we will look at:
To understand the epigenome is to see life’s software in action, the living instructions that tell the body not just what to be, but when and how to be it. And in learning how it changes, we may uncover the keys to not just slowing aging, but re-writing parts of the story itself.
Let us step into this library together, and see what chapters might yet be restored.
Imagine your DNA as the master blueprint for building an entire house, the house that is you.
This blueprint contains every instruction needed to construct every wall, every window, every piece of wiring and plumbing.
Each room in this house, just like your cells, has a specific function, so it doesn’t need to read the entire blueprint.
The kitchen doesn’t need instructions for laying carpet in the bedroom, and the bathroom doesn’t care about wiring the living room lights.
This is where epigenetic alterations come into play.
Epigenetics is the system that ensures each room only opens the right pages of the blueprint, telling a muscle cell to activate “muscle instructions,” a nerve cell to activate “nerve instructions,” and so on, while keeping the rest of the blueprint securely closed.
But how does this work at the molecular level?
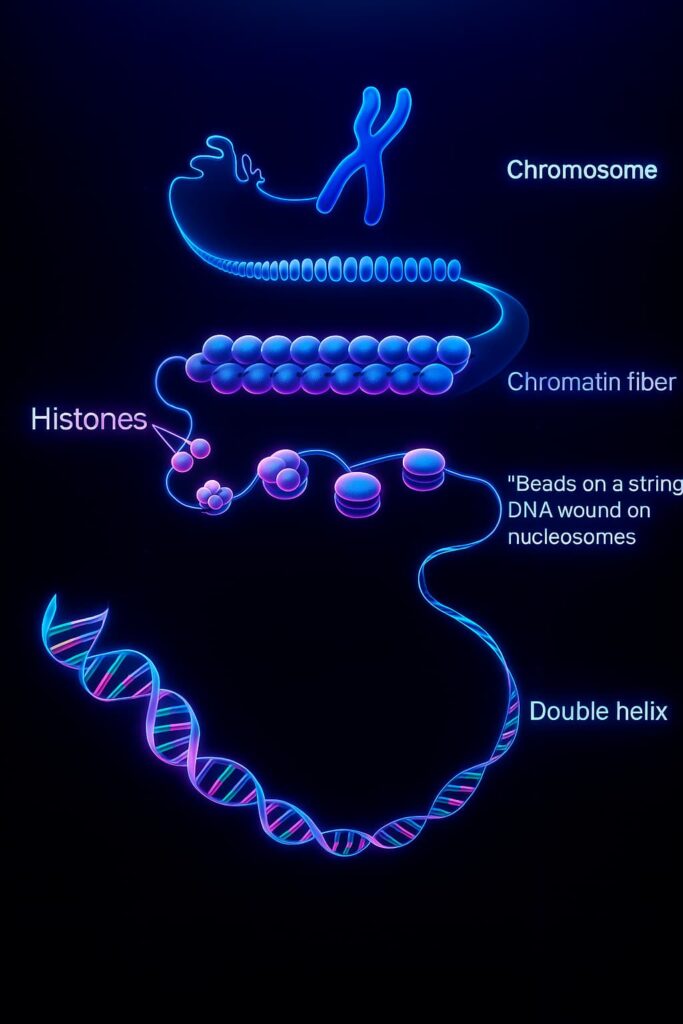
Your DNA isn’t just lying around loosely in the nucleus, it’s tightly packed into structures called chromatin.
Picture the blueprint rolled up into scrolls so it can all fit inside the tiny nucleus of each cell.
This packing is done by special proteins called histones, which act like spools around which the DNA winds itself.
When DNA is tightly wound around histones, it’s like a locked-up scroll ,difficult to read and mostly silent.
But your cells need to unpack certain parts of the blueprint to access the instructions and function properly.
This is where epigenetic marks, and the process of epigenetic alterations, come in.
These chemical “post-it notes” tell the cell which sections to keep locked up and which to open for reading.
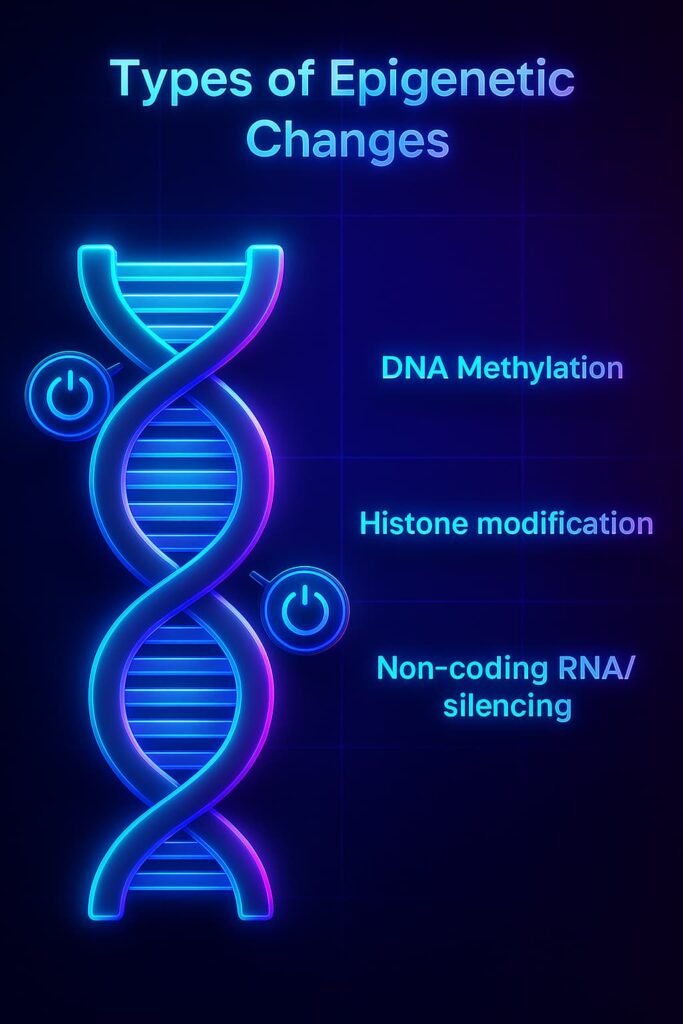
Epigenetic alterations work at three main levels:
1. Histone modification: chemical changes to the histones themselves that loosen or tighten how much DNA is wrapped around them.
When histones loosen their grip, DNA unwinds and becomes readable.
When they clamp down tightly, DNA stays coiled and unreadable.
2. DNA methylation: tiny chemical tags called methyl groups (a carbon atom with three hydrogens, CH₃) attach to DNA, especially near gene “on/off” switches called promoters.
When a promoter is heavily methylated, it’s like putting a big “Do Not Enter” sign on that section of the blueprint: the cell skips it and the gene remains silent.
3. Non-coding RNA silencing: small molecules called non-coding RNAs (like microRNAs) act as guides that help silence specific genes by interfering with how their messages are read or processed.
It’s like having a librarian who notices an unwanted chapter being copied and quietly intercepts the pages before they can be used, ensuring certain parts of the blueprint remain unread when they shouldn’t be active.
Together, these processes control gene expression, ensuring each room (or cell) reads only the correct part of the master blueprint while keeping the rest tucked away.
This precise regulation allows your body to maintain over 200 specialized cell types, all sharing the same DNA sequence but expressing different sets of genes depending on their role, thanks to the fine-tuned settings of the epigenome and the impact of epigenetic alterations.
Over time, our beautifully choreographed system of gene control begins to slip, a gradual process known as epigenetic alterations.
When we’re young, the epigenetic instructions are sharp and orderly. Each cell reads only the genes it needs, locking the rest away. But as we age, those molecular bookmarks, like DNA methylation marks and histone tags start to fade, land in the wrong places, or disappear entirely. The result? The blueprint gets harder to read.
So what causes this shift in the molecular software of our cells?
1. Oxidative Stress: The Rust of Aging
Oxidative stress is like rust on your cellular blueprint. Reactive oxygen species (ROS), unstable molecules produced during metabolism, especially by dysfunctional mitochondria, build up over time and attack both DNA and the enzymes that maintain epigenetic stability.
These attacks disrupt enzymes like DNA methyltransferases and histone modifiers, leading to misplaced or lost chemical tags. The result? A gradual breakdown in the precision of your epigenetic program, one of the first cracks in the foundation of aging and epigenetics.
2. Chronic Inflammation: The Constant Alarm
Now imagine trying to file documents while a fire alarm blares nonstop.
That’s chronic inflammation, or inflammaging. Pro-inflammatory molecules like cytokines disrupt the enzymes that control DNA methylation and histone modification, flipping genes on or off at the wrong time. This chaos accelerates both inflammation and epigenetic drift, forming a damaging loop.
3. Imperfect Epigenetic Copying
Every time a cell divides, it must replicate not just its DNA, but the entire epigenetic landscape. It’s not just copying the words, it’s replicating the highlighting, footnotes, and bookmarks.
But these marks are harder to copy than raw code. Over many divisions, errors creep in: missing methyl groups, scrambled histone tags, and incomplete reassembly of the original structure. The result? Cells start making “fuzzy photocopies” of themselves and that confusion builds over time.
4. Environmental Damage: Graffiti on the Genome
Finally, life itself takes a toll.
Pollution, cigarette smoke, pesticides, processed foods, and heavy metals all damage DNA and interfere with epigenetic enzymes. They can deplete methyl donors like folate and B vitamins or add random chemical tags like graffiti on your cellular blueprint.
Together, these factors drive one of the most fundamental hallmarks of aging: epigenetic alterations. They don’t just reflect wear and tear, they represent a dynamic, cumulative breakdown of gene control, driven by both internal stress and environmental exposure.
As this software becomes scrambled, cells lose their identity, tissues lose function, and the body’s ability to repair and adapt slowly fades, pulling us deeper into the biology of aging.
As we’ve seen, epigenetic alterations are more than just misplaced chemical tags or messy molecular “graffiti.” They represent a slow but steady collapse of the finely tuned software that once kept your cells running smoothly.
And this cellular confusion comes with serious consequences.
1. Cells lose their identity
When epigenetic regulation falters, cells can literally forget who they are. A skin cell might start expressing liver genes, or a muscle cell might silence critical functions. The result? Dysfunctional cells acting out of sync like a kitchen trying to be a bathroom.
2. Stem cells lose their power
Your stem cells, the regenerative engine of your body, rely on precise epigenetic settings to stay youthful and responsive. But as epigenetic drift accumulates, they struggle to divide, specialize, or repair damage. This weakens tissue regeneration and accelerates aging.
3. Inflammation ramps up
Aging and epigenetics are closely tied to inflammation. Altered gene expression can mistakenly activate immune pathways, triggering a constant low-level “alarm” throughout the body. This inflammaging damages tissues and fuels other hallmarks of aging, like mitochondrial dysfunction and senescence.
4. Disease risk rises
One of the most dangerous effects of epigenetic alterations? They can silence protective genes (like tumor suppressors) or activate harmful ones (like oncogenes). The result: increased vulnerability to cancer, metabolic disorders, and neurodegeneration.
As the harmony between your DNA and its epigenetic tags unravels, cells misfire, tissues falter, and the body loses its ability to adapt and repair.
This isn’t just aging. it’s a breakdown in your biological software.
Fortunately, emerging tools in cellular reprogramming and longevity science are showing promise in resetting these faulty instructions… and helping your cells remember how to be young again.
Your epigenome isn’t locked in. It’s more like a chalkboard than a stone tablet, constantly rewritten in response to your environment, behavior, and biology. That means you’re not just a passenger on the aging train, your daily habits steer the ride.
While epigenetic alterations accumulate with time, certain lifestyle choices and nutrients can help maintain balance, slow the drift, and support long-term epigenetic health.
1. Eat an epigenetic-friendly diet
Nutrients called methyl donors, including folate, B vitamins, and choline, are essential for maintaining DNA methylation. They help cells tag the right genes at the right time, preserving healthy gene expression.
2. Exercise consistently
Physical activity reduces inflammation, improves metabolic control, and supports beneficial epigenetic changes. It’s like sending a repair crew into your genetic library to clean up disorganized shelves.
3. Manage chronic stress
Ongoing stress can disrupt epigenetic regulation, silencing repair genes and activating inflammatory pathways. Techniques like meditation, deep breathing, and time in nature help maintain internal balance.
4. Get restorative sleep
During sleep, your body performs key tasks, from repairing oxidative damage to reorganizing epigenetic tags. Poor sleep accelerates epigenetic aging and disrupts gene expression patterns.
If your diet or lifestyle falls short, certain supplements may offer extra support, giving your cells the raw materials and regulatory signals they need to maintain a healthy epigenetic profile.
1. Folate and B vitamins (B6, B12)
These are critical methyl donors, nutrients your body uses to apply and maintain methylation tags on DNA. Adequate intake keeps your cellular blueprint organized and responsive.
2. Choline
Choline supports methylation pathways and is especially important for brain and liver health. Low levels can disrupt epigenetic programming and cognitive resilience as you age.
3. Omega-3 fatty acids (EPA & DHA)
Found in fish oil and algae, omega-3s reduce inflammation and have been shown to influence epigenetic gene expression related to metabolic health and aging. They may also support brain function and cardiovascular resilience.
4. Polyphenols: resveratrol & curcumin
These plant compounds help regulate the “writers” and “erasers” of the epigenome, enzymes that modify histones and methylation.
Not medical advice. Always check with your doctor before using any supplement.
Final Takeaway
While aging and epigenetic alterations are natural, they’re not entirely out of your control.
By combining healthy daily habits with strategic nutrient support, you can help preserve the software that runs your cells, protecting your identity at the molecular level and unlocking real potential in longevity science.
For decades, we’ve talked about epigenetic drift as a one-way street. But what if we could actually reverse it, restoring youthful epigenetic patterns and reprogramming aging cells to act young again?
That’s the promise of partial reprogramming.
This approach uses a set of genes called the Yamanaka factors, four powerful regulators discovered by Nobel laureate Shinya Yamanaka. When fully activated, they can return adult cells to an embryonic-like state, resetting epigenetic marks and rejuvenating cell function.
But fully reprogramming a cell erases its identity, not ideal for, say, a functioning heart or skin cell.
So scientists are now testing partial reprogramming, gently activating these factors just enough to reverse age-related epigenetic alterations without turning cells into stem cells. It’s like a careful software update: refreshing performance without wiping the hard drive.
In early animal studies, the results are stunning:
This could revolutionize how we think about aging, not just as damage, but as a loss of epigenetic information that might be restorable.
While we’re still in the early stages, this research opens the door to something profound: a future where cellular reprogramming helps us reset aging at the molecular level.
It’s not just about damaged DNA, it’s about how your cells read it.
That’s where epigenetic alterations come in. These tiny chemical tags tell your genes when to turn on, off, or chill like highlighters on your genetic script. But over time, stress, toxins, poor sleep, and even normal cell division scramble those tags. The result? Confused cells, sluggish repair, rising inflammation, and a body that forgets how to stay young.
The good news? Your epigenome isn’t carved in stone, it’s more like a chalkboard.
With the right daily habits: nutrient-rich foods, movement, deep sleep, stress management. you can help keep your gene expression sharp and youthful. And with smart supplements like B vitamins, omega-3s, and polyphenols, you can give your cells the tools to stay on track.
And
And the cutting-edge bit is that scientists are learning how to reprogram those aging cells, resetting their epigenetic software to a younger state. It’s not sci-fi. It’s happening.
Because aging isn’t just wear and tear, it’s a loss of information.
And guess what? We might just be learning how to restore it.
Now that you’ve unlocked the science behind Epigenetic Alterations, why stop here? The aging process is a mosaic and Epigenetic Alterations is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?