Science Meets Longevity
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

Journey Into the Biology of Time
I’ve been asking the same question for a long time:
Why do we age, and can we fight it?
This site is my way of chasing the answers and sharing the tools, insights, and science I find along the way.

“Telomeres don’t just mark the passage of time, they help define the pace of aging itself.”
Welcome, fellow traveler in the landscape of time.
If you are here, you have likely felt the quiet pull of one of biology’s most poetic truths: hidden at the ends of your DNA are tiny guardians of your youth, the telomeres. These delicate protective caps are like the bindings on the book of life, holding your genetic story together each time a cell divides.
But with every chapter turned, every renewal of your cells, the bindings wear thin. Bit by bit, the telomeres shorten, and when they become too frayed, the pages of the book can no longer be copied without error. The story falters. The cell withdraws from the cycle of life, slipping into dysfunction or death.
This quiet erosion is not merely a curiosity. It is officially recognized as one of the hallmarks of aging, a fundamental driver of cellular decline, tissue damage, and the gradual dimming of vitality. The shortening of telomeres is a clock ticking within us all, counting down not just years, but the capacity of our cells to repair, renew, and thrive.
In this journey, we will explore:
We will also see how telomere loss intertwines with other forces of aging, like cellular senescence, where damaged cells linger and disrupt their neighbors, stirring up inflammation and accelerating decline.
To understand telomeres is to glimpse one of life’s most elegant clocks, a timer written into the fabric of every cell. And while we may not yet know how to stop it, we are learning ways to slow its ticking, to stretch the chapters of health before the final page.
Let us turn that page together, and see what the science has to reveal.
Imagine your chromosomes: a long, tightly packed strands of DNA stored inside the nucleus of nearly every cell in your body. These chromosomes carry your entire genetic blueprint, 46 total, with 23 from your mother and 23 from your father, encoding the instructions that make you, well… you.
Now picture each chromosome like a shoelace.
At the very ends of those shoelaces? That’s where telomeres come in.
These telomeres are protective caps made up of repeating DNA sequences. in humans, it’s the pattern TTAGGG, repeated thousands of times. They don’t contain genes or build proteins. instead, they act like buffers, shielding the rest of your DNA from damage.
Why does that matter? Because every time a cell divides, it can’t quite copy the very ends of its DNA, a phenomenon called the end-replication problem. So with each division, a little bit of telomere gets shaved off.
By sacrificing a small piece of themselves, telomeres prevent the loss of essential genetic code like bumpers taking the hit so the car stays intact.
So where is the problem? telomeres don’t last forever.
As they shorten with each round of cell division, the protection wears thin. Eventually, cells hit a limit and that’s when trouble starts. Essential DNA begins to degrade, leading to cellular malfunction, mutations, and an increased risk of errors… all of which accelerate the aging process.
This slow erosion of telomeres is deeply tied to telomeres and aging and is one of the earliest contributors to genomic instability, a hallmark that disrupts the very structure and function of our DNA over time.
That’s why there’s so much excitement around emerging tools like telomerase therapy and cellular reprogramming technologies that may one day help preserve telomere length, protect chromosome integrity, and extend healthy lifespan.
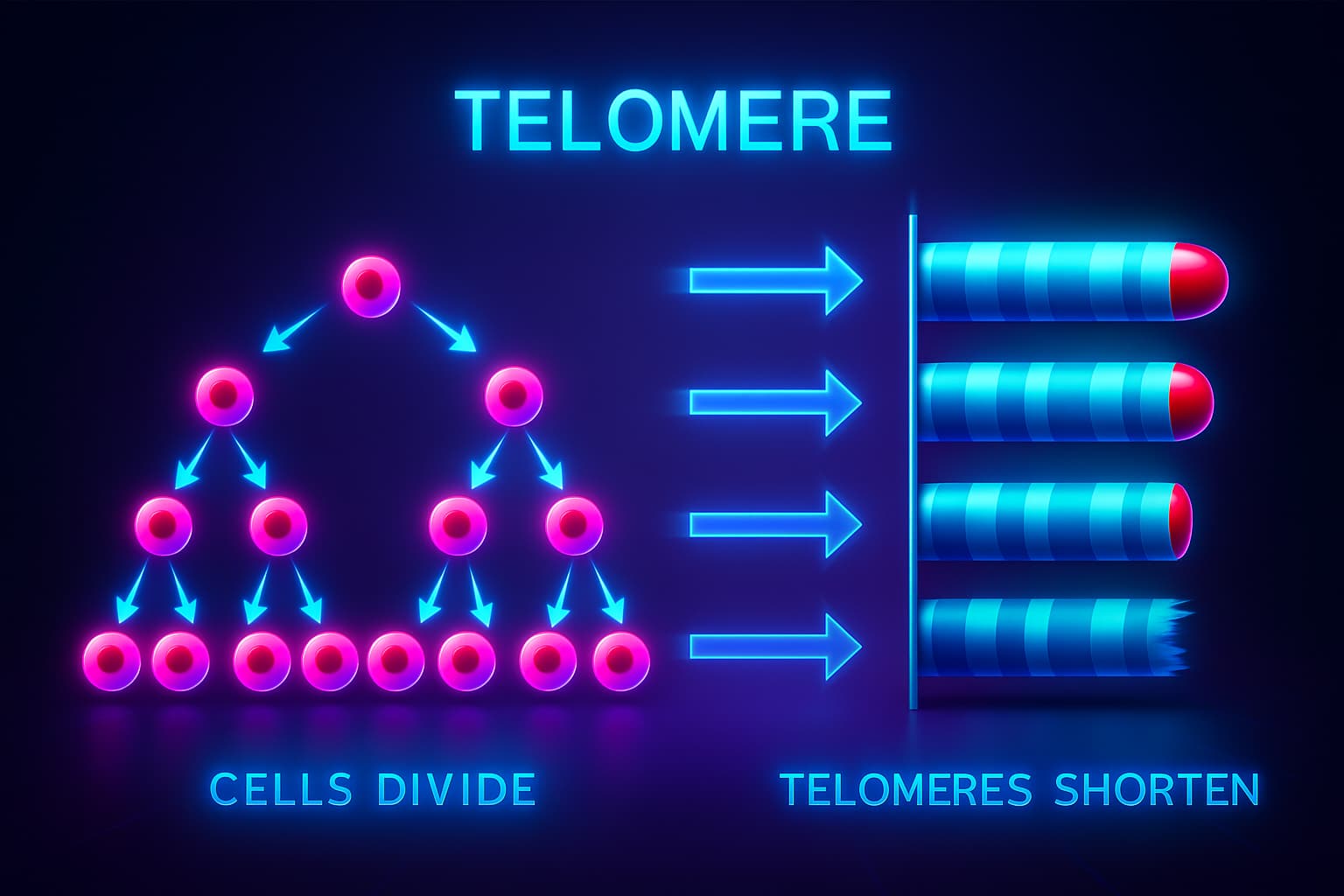
The cells in your body are constantly dying, that’s just a fact of life.
But don’t worry: your body has a backup plan. When you’re young, you have plenty of stem cells, your biological reservoir, ready to divide and replenish the cells you lose every day.
Every time a stem cell divides, it must perfectly copy its entire DNA, an incredibly precise but imperfect process.
The Replication Flaw That Ages Us:
The enzyme that copies DNA, called DNA polymerase, is like an old printer that can’t quite print to the very edge of the page. It requires a primer: a short stretch of RNA, like the first brick that gives the builder a place to start laying bricks and can only work in one direction (from the 5’ end to the 3’ end).
Since DNA is double-stranded, both strands must be copied together but only one strand, the leading strand, is copied smoothly. The other, called the lagging strand, is copied in short sections known as Okazaki fragments.
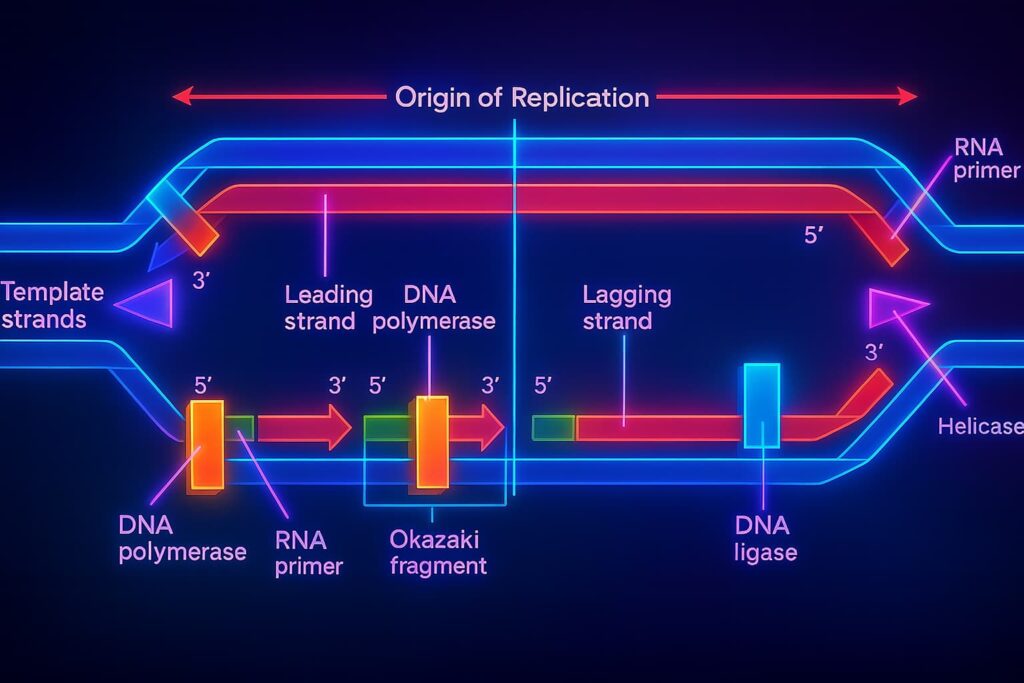
This awkward process creates a critical flaw:
At the very end of the lagging strand, the last primer sits at the tip. Because primers are made of RNA and not DNA, they are removed at the end of replication so they can be properly replaced with DNA. But at the very tip, once the final primer is removed, there’s no upstream DNA available to fill in that gap. As a result, a small section at the end remains uncopied, leading to gradual telomere shortening every time the cell divides.
Fortunately, evolution provided a solution: telomeres. These protective caps are made of repetitive, non-coding DNA (TTAGGG repeats) that act as expendable buffers. They protect your telomeres by absorbing this inevitable shortening so your essential genes remain safe.
But this protective buffer isn’t infinite.
With every cell division, telomeres get shorter. Eventually, when they shrink too much, the cell recognizes this as a danger signal and stops dividing, either entering senescence (a dormant, “retirement” state) or triggering apoptosis (programmed cell death).
This progressive telomere shortening is at the heart of telomeres and aging, and it explains why scientists are exploring promising approaches like telomerase therapy and cellular reprogramming for longevity as ways to restore telomere length and extend healthy lifespan.
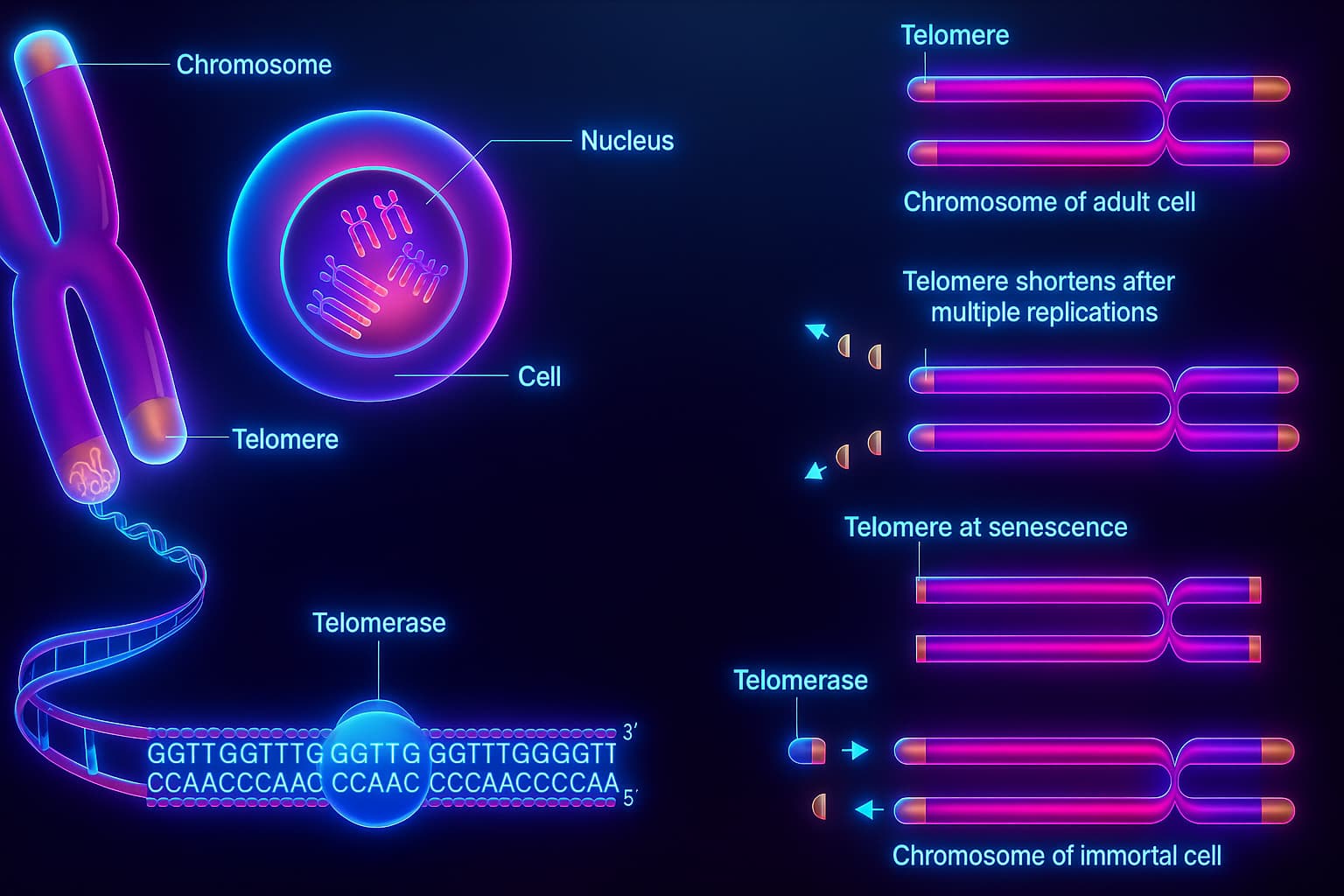
At birth, your telomeres are long, healthy, and full of potential, giving your cells the ability to divide, regenerate, and keep your tissues vibrant.
But as we age, that potential gradually fades.
Each time a stem cell divides, its telomeres get a little shorter, until eventually, they’re too short to protect the chromosome ends. This slow, predictable erosion is so closely tied to aging that scientists often refer to it as a “molecular clock”. ticking down with every round of cell division.
It’s like a biological hourglass, flipped the moment we’re born.
Grain by grain, telomere loss tracks the passage of time in our cells.
When telomeres become short, the effects ripple through the body:
In short: telomere shortening limits the lifespan of our cells and ultimately, our bodies.
That’s why understanding this connection between telomeres and aging has become such a hotbed of research. It’s fueling new interest in how we might protect telomeres through lifestyle and nutrition and explore cutting-edge interventions like telomerase therapy and cellular reprogramming to preserve or even restore our cellular clocks.telomere length to promote healthier aging.
Now you might be wondering:
“If telomeres shorten every time a cell divides, how do embryonic cells maintain such long telomeres? There must be a way to reset them!”
Well… there is.
The key player is an enzyme called telomerase.
Telomerase can actually rebuild telomeres by adding back those repetitive DNA sequences, effectively restoring the protective caps. In theory, this means a cell could keep dividing indefinitely, almost like a biological fountain of youth!
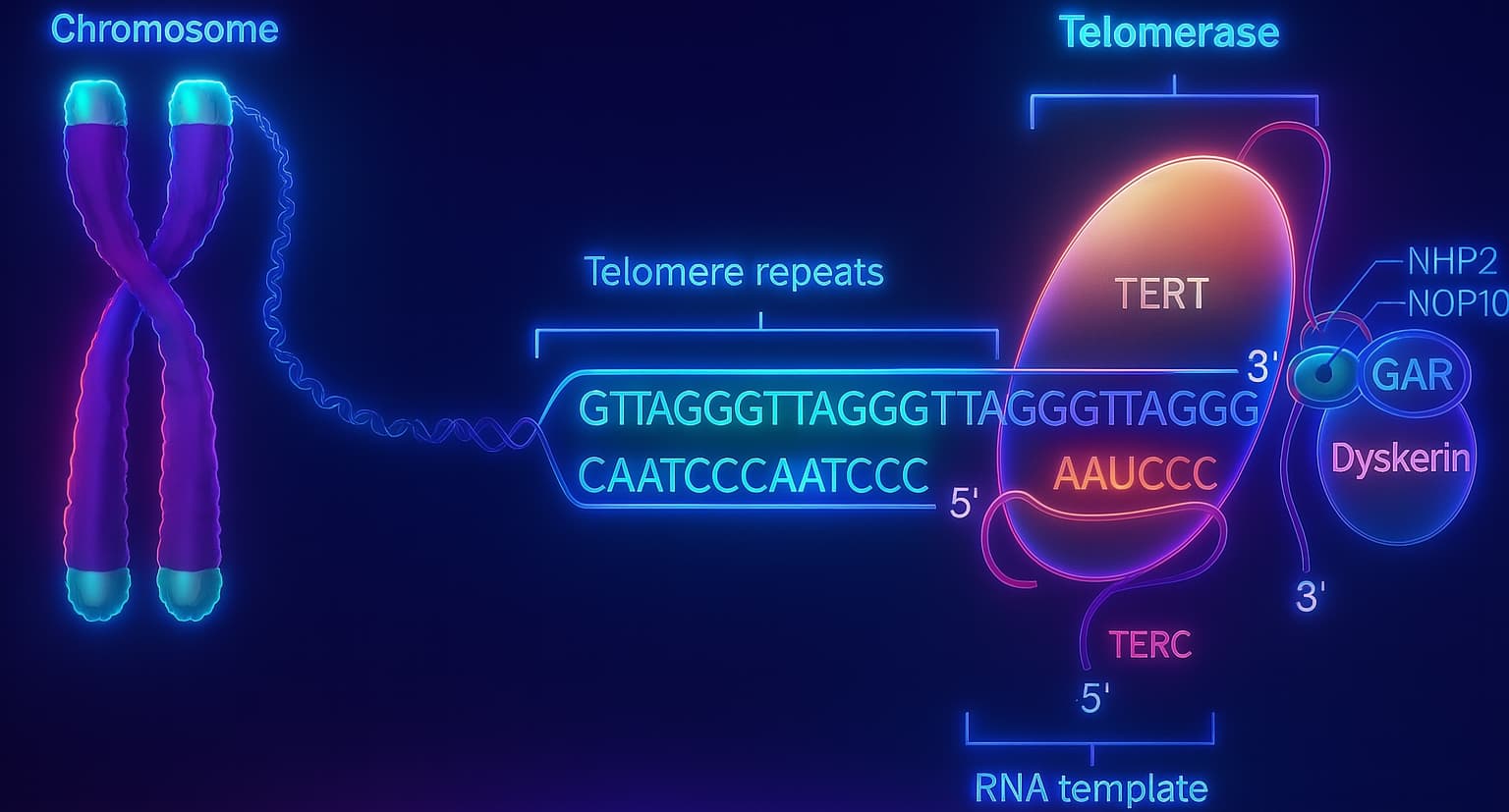
But there’s a twist.
In embryonic cells, telomerase is fully active, allowing rapid and repeated cell division during development without much telomere loss, essential for early growth.
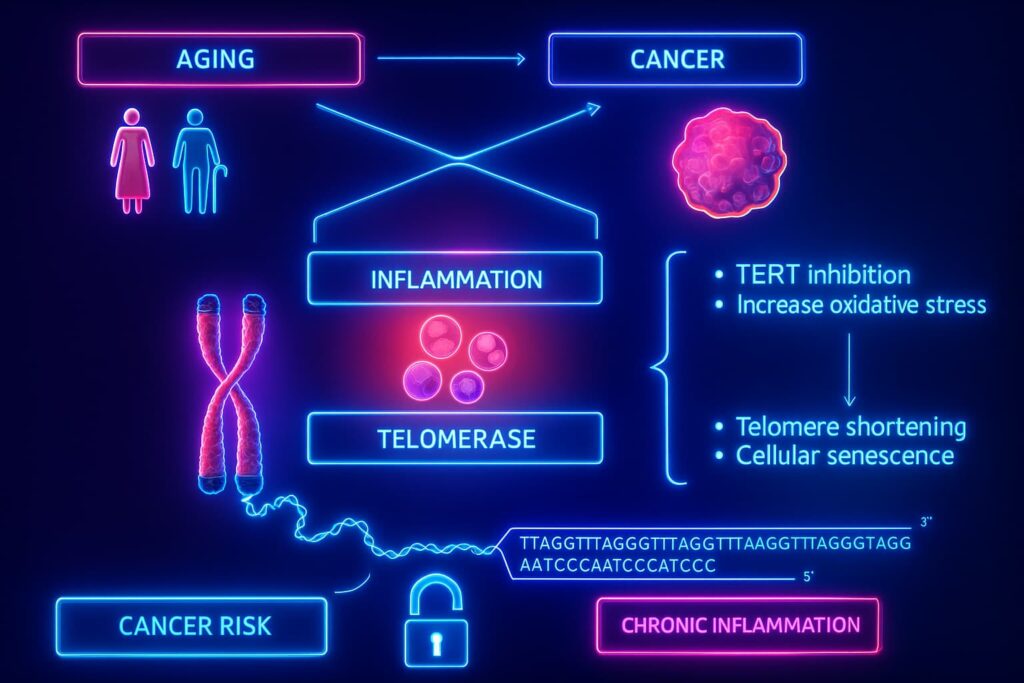
In adult cells, however, telomerase is mostly shut down. Why? Because if telomerase stayed active, damaged cells could keep dividing endlessly, a recipe for cancer. Uncontrolled telomerase activity is one of cancer’s tricks for escaping normal growth limits.
So evolution struck a compromise: reduce cancer risk by limiting telomerase, at the cost of gradual telomere shortening and reduced tissue regeneration as we age.
This trade-off is at the heart of the link between telomeres and aging and why therapies like telomerase activation and cellular reprogramming are being explored to restore telomere length safely.
Fun fact? Lobsters keep telomerase active in many of their cells for life. They show few signs of aging and can live over a century, a striking example of how telomere protection may promote longevity.
The good news? While we can’t completely stop telomere shortening, it’s a natural part of how cells divide, we can slow it down.
Research shows that smart lifestyle habits can help protect telomeres by reducing two major drivers of damage: oxidative stress and chronic inflammation.
Oxidative stress occurs when reactive oxygen species (ROS) build up in the body. These unstable molecules can damage DNA and telomeres, with their repetitive structure, are especially vulnerable. ROS make it harder for cells to copy the chromosome ends correctly, leading to faster telomere erosion.
Chronic inflammation makes things worse. An overactive immune system causes cells to divide more often, draining telomeres, while inflammation itself increases oxidative stress. This cycle doesn’t just affect telomeres; it also contributes to other aging mechanisms like loss of proteostasis and immune fatigue.
Fortunately, the body has a backup system, telomerase: an enzyme that can rebuild telomeres by adding DNA back to their ends. But in most cells, telomerase activity fades with age. Only stem cells and a few specialized types keep it active long-term.
That’s where your daily choices come in.
By lowering inflammation and reducing oxidative stress through targeted habits and nutrients, you can help protect your telomeres and give your cells a better shot at longevity.
If you’re wondering how to protect telomeres naturally, start here:
1. Manage stress
Chronic stress increases cortisol, inflammation, and oxidative stress all of which speed up telomere shortening. Oxidative stress produces harmful molecules that can directly damage the DNA at chromosome ends, while inflammation accelerates cell turnover, draining your telomeres’ protective buffer.
2. Eat an antioxidant-rich diet
Fruits, vegetables, nuts, seeds, and whole grains are packed with antioxidants like vitamin C, vitamin E, and polyphenols. These compounds help neutralize free radicals before they can attack your DNA, supporting telomere maintenance over time.
3. Exercise regularly
Moderate, consistent exercise reduces chronic inflammation, boosts antioxidant defenses, and enhances DNA repair, all factors that help protect telomeres.
While intense workouts can temporarily increase oxidative stress, the long-term benefits for telomere health are clear.
4. Get enough sleep
Sleep is your body’s nightly repair mode, including DNA repair.
Poor sleep is linked to increased inflammation and oxidative stress, contributing to faster telomere shortening and accelerated cellular aging.
5. Avoid smoking and excessive alcohol
Toxins from smoking and alcohol metabolism (e.g., acetaldehyde) promote oxidative damage and chronic inflammation, directly harming telomeres and aging your cells faster.
While no supplement can “regrow” lost telomeres overnight, some nutrients and compounds show promise in reducing the underlying stresses that shorten them, potentially supporting telomere length and slowing biological aging.
1. Omega-3 fatty acids
Found in fish oil, flaxseeds, and walnuts, omega-3s reduce inflammation and oxidative stress, two major drivers of telomere shortening.
2. Vitamin D
Vitamin D supports immune balance and helps keep chronic inflammation in check, which may reduce telomere wear and promote healthier aging.
3. Polyphenols (resveratrol, curcumin)
These plant-based antioxidants protect cells from free radical damage. Resveratrol (from red wine and grapes) and curcumin (from turmeric) activate pathways that reduce oxidative stress, which may help protect your telomeres from accelerated shortening.
4. Astragalus extracts
Astragalus is especially intriguing:
Some extracts from this traditional herb have been shown in early research to stimulate telomerase activity, the very enzyme that can rebuild telomeres.
In theory, this could help extend the replicative lifespan of certain cells, a concept at the heart of telomerase therapy.
However, this research is still preliminary, and much more is needed before any conclusions can be made for humans.
Not medical advice. Always check with your doctor before using any supplement.
We’ve seen how telomeres act like a biological hourglass, ticking down as cells divide. But what if we could flip the timer? Or even add sand back in?
That’s exactly what emerging science is exploring through two powerful ideas: cellular reprogramming and telomerase therapy.
1. Cellular Reprogramming: Rewinding the Clock
In a groundbreaking discovery, Nobel laureate Shinya Yamanaka showed that by introducing just four genes, the now-famous Yamanaka factors, we can reset adult cells to a youthful, embryonic-like state.
What’s astonishing is that this process also appears to restore telomere length, rejuvenating the cell’s protective caps. It’s as if a worn-out page of a book is smoothed out and made new again.
This launched a revolution in regenerative medicine, sparking new strategies to reverse aging markers, including telomere shortening without fully erasing cellular identity.
2. Telomerase Therapy: Restoring the Enzyme
Telomerase is the enzyme that rebuilds telomeres, active in stem cells and embryos, but mostly silent in adult tissues.
Telomerase therapy aims to reactivate this enzyme by delivering its gene (TERT) into aging cells, often using viral vectors. The idea: extend the healthy lifespan of cells by rebuilding their telomeres.
In animal studies, mice treated with telomerase therapy lived longer, healthier lives, with no increased cancer risk when carefully controlled. The potential? Stronger tissues, delayed degeneration, and extended healthspan.
But there’s a caveat: cancer cells also use telomerase to divide endlessly. So this therapy must be approached with extreme precision to avoid triggering uncontrolled growth.
3. The Frontier of Telomere Science
These ideas aren’t science fiction, they’re already being tested in labs. Partial reprogramming techniques are being developed to reverse aging features without reverting cells to a risky embryonic state.
While we don’t have a reset button yet, cellular reprogramming for longevity and telomerase therapy are two of the most promising tools in aging science, revealing just how adaptable biology can be.
Telomeres, those tiny caps at the ends of our chromosomes, may seem like small players, but they have an outsized impact on our health, longevity, and the aging process.
With every cell division, telomeres shorten, gradually ticking down the clock of cellular life. When they become critically short, cells can no longer divide safely, leading to tissue decline, reduced regeneration, and many of the signs we associate with aging.
But there is some good news:
While we can’t stop telomere shortening entirely, how we live matters.
By reducing oxidative stress, lowering chronic inflammation, avoiding toxins, eating antioxidant-rich foods, exercising regularly, and managing stress, we can help slow this biological countdown and better protect our telomeres.
Even more exciting are scientific breakthroughs pointing to future therapies, from natural compounds like astragalus extracts, which may stimulate telomerase activity, to cutting-edge innovations like cellular reprogramming for longevity using Yamanaka factors and experimental telomerase therapy aimed at rebuilding and restoring telomere length directly.
In short:
Telomeres are both a marker and a driver of biological aging. By understanding their role and learning how to protect them, we take a meaningful step toward keeping our cells, and ourselves, healthier for longer.
Now that you’ve unlocked the science behind Telomere Shortening and aging, why stop here? The aging process is a mosaic and Telomere Shortening is just one piece.
Explore the other hallmarks of aging and see how they connect, interact, and build the bigger picture of biological aging and longevity. Pick the ones that spark your curiosity:
Each of these threads tells a different part of the aging story and each one offers a chance to intervene, repair, and thrive longer.
So… which one will you explore next?